Functional characterization and target validation of alternative complex I of Plasmodium falciparum mitochondria
- PMID: 16641458
- PMCID: PMC1472221
- DOI: 10.1128/AAC.50.5.1841-1851.2006
Functional characterization and target validation of alternative complex I of Plasmodium falciparum mitochondria
Abstract
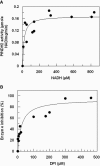
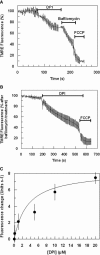
This study reports on the first characterization of the alternative NADH:dehydrogenase (also known as alternative complex I or type II NADH:dehydrogenase) of the human malaria parasite Plasmodium falciparum, known as PfNDH2. PfNDH2 was shown to actively oxidize NADH in the presence of quinone electron acceptors CoQ(1) and decylubiquinone with an apparent K(m) for NADH of approximately 17 and 5 muM, respectively. The inhibitory profile of PfNDH2 revealed that the enzyme activity was insensitive to rotenone, consistent with recent genomic data indicating the absence of the canonical NADH:dehydrogenase enzyme. PfNDH2 activity was sensitive to diphenylene iodonium chloride and diphenyl iodonium chloride, known inhibitors of alternative NADH:dehydrogenases. Spatiotemporal confocal imaging of parasite mitochondria revealed that loss of PfNDH2 function provoked a collapse of mitochondrial transmembrane potential (Psi(m)), leading to parasite death. As with other alternative NADH:dehydrogenases, PfNDH2 lacks transmembrane domains in its protein structure, and therefore, it is proposed that this enzyme is not directly involved in mitochondrial transmembrane proton pumping. Rather, the enzyme provides reducing equivalents for downstream proton-pumping enzyme complexes. As inhibition of PfNDH2 leads to a depolarization of mitochondrial Psi(m), this enzyme is likely to be a critical component of the electron transport chain (ETC). This notion is further supported by proof-of-concept experiments revealing that targeting the ETC's Q-cycle by inhibition of both PfNDH2 and the bc(1) complex is highly synergistic. The potential of targeting PfNDH2 as a chemotherapeutic strategy for drug development is discussed.
Figures




References
-
- Allen, R. J., and K. Kirk. 2004. The membrane potential of the intraerythrocytic malaria parasite plasmodium falciparum. J. Biol. Chem. 279:11264-11272. - PubMed
-
- Berenbaum, M. C. 1978. A method for testing for synergy with any number of agents. J. Infect. Dis. 137:122-130. - PubMed
-
- Biagini, G. A., D. Lloyd, K. Kirk, and M. R. Edwards. 2000. The membrane potential of Giardia intestinalis. FEMS Microbiol. Lett. 192:153-157. - PubMed
-
- Biagini, G. A., P. M. O'Neill, A. Nzila, S. A. Ward, and P. G. Bray. 2003. Antimalarial chemotherapy: young guns or back to the future? Trends Parasitol. 19:479-487. - PubMed
-
- Bjorklof, K., V. Zickermann, and M. Finel. 2000. Purification of the 45 kDa, membrane bound NADH dehydrogenase of Escherichia coli (NDH-2) and analysis of its interaction with ubiquinone analogues. FEBS Lett. 467:105-110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

