Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging
- PMID: 16641903
- PMCID: PMC2361284
- DOI: 10.1038/sj.bjc.6603116
Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging
Abstract
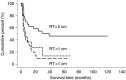
A retrospective analysis was performed to evaluate the clinical characteristics and prognostic factors in the patients with clear cell carcinoma (CCC) of the ovary. After central pathological review and scanning of the medical records of nine Japanese institutions between 1992 and 2003, a total of 254 patients with CCC of the ovary were enrolled in the present study. Mean age was 52.4 years (range 23-73 years). Tumours were 13% (33/254) stage Ia, 36% (92/254) stage Ic, 13% (33/254) stage II, 30% (80/254) stage III, and 6% (16/254) stage IV. Five-year progression-free survival and overall survival was 84 and 88% in stage I, 57 and 70% in stage II, 25 and 33% in stage III and 0 and 0% in stage IV, respectively. Retroperitoneal lymph node metastasis was observed in 9% in pT1a tumours, 7% in pT1c tumours, 13% in pT2 tumours, and 58% in pT3 tumours, respectively. There was no survival benefit according to chemotherapeutic differences in the patients who received complete surgical staging procedures and conventional chemotherapy. Peritoneal cytological status was an independent prognostic factor in stage Ic patients (P=0.03) and only residual tumour diameter was an independent prognostic factor in stage III, IV patients (P=0.02). Our results suggest that cytoreductive surgery resulting in no residual tumour only could improve the prognosis of advanced CCC patients.
Figures


References
-
- Adachi S, Ogasawara T, Yamasaki N, Shibahara H, Kanazawa R, Tsuji Y, Takemura T, Koyama K (1999) A pilot study of CPT-11 and cisplatin for ovarian clear cell adenocarcinoma. Jpn J Clin Oncol 29: 434–437 - PubMed
-
- Behbakht K, Randall TC, Benjamin I, Morgan MA, King S, Rubin SC (1998) Clinical characteristics of clear cell carcinoma of the ovary. Gynecol Oncol 70: 255–258 - PubMed
-
- Bray F, Loos AH, Tognazzo S, La Vecchia C (2005) Ovarian cancer in Europe: cross-sectional trends in incidence and mortality in 28 countries, 1953–2000. Int J Cancer 113: 977–990 - PubMed
-
- Cass I, Li AJ, Runowicz CD, Fields AL, Goldberg GL, Leuchter RS, Lagasse LD, Karlan BY (2001) Pattern of lymph node metastases in clinically unilateral stage I invasive epithelial ovarian carcinomas. Gynecol Oncol 80: 56–61 - PubMed
-
- Crozier MA, Copeland LJ, Silvia EG, Gershenson DM, Stringer CA (1989) Clear cell carcinoma of the ovary: a study of 59 cases. Gynecol Oncol 35: 199–203 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

