Docetaxel-based induction therapy prior to radiotherapy with or without docetaxel for non-small-cell lung cancer
- PMID: 16641904
- PMCID: PMC2361263
- DOI: 10.1038/sj.bjc.6603115
Docetaxel-based induction therapy prior to radiotherapy with or without docetaxel for non-small-cell lung cancer
Abstract
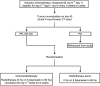
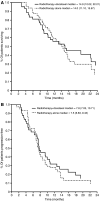
This trial aimed to assess the feasibility and tumour control of concurrent chemoradiotherapy or radiotherapy alone after docetaxel-based induction chemotherapy in locally advanced non-small-cell lung cancer (NSCLC). Patients with stage IIIA/IIIB NSCLC received two 21-day cycles of induction chemotherapy with docetaxel (85 mg m(-2), day 1) plus cisplatin (40 mg m(-2), days 1 and 2). Patients without disease progression on day 43 were randomised to radiotherapy (2 Gy for 5 days week(-1); total 60 Gy) alone or with docetaxel 20 mg m(-2) once weekly every 6 weeks. Of 108 patients who received induction chemotherapy, 104 were evaluable for response. After induction chemotherapy, the overall response rate (ORR) was 44%; 91 (88%) patients had no disease progression and 89 were subsequently randomised to local treatment. After randomised therapy, the ORR was 53% (chemoradiotherapy 58%; radiotherapy 48%). Median survival and time to progression were 14.9 and 7.8 months, respectively, for chemoradiotherapy and 14.0 and 7.5 months, respectively, for radiotherapy. The most common toxicities during induction chemotherapy and randomised therapy were grades 3-4 neutropenia and grade 3 lymphocytopenia, respectively. Docetaxel-cisplatin induction therapy followed by concurrent docetaxel and thoracic radiotherapy is a feasible treatment option, showing good clinical activity and tolerability, for locally advanced NSCLC.
Figures
References
-
- Betticher DC, Hsu Schmitz SF, Totsch M, Hansen E, Joss C, von Briel C, Schmid RA, Pless M, Habicht J, Roth AD, Spiliopoulos A, Stahel R, Weder W, Stupp R, Egli F, Furrer M, Honegger H, Wernli M, Cerny T, Ris HB (2003) Mediastinal lymph node clearance after docetaxel–cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J Clin Oncol 21: 1752–1759, doi:10.1200/JCO.2003.11.040 - PubMed
-
- Creane M, Seymour CB, Colucci S, Mothersill C (1999) Radiobiological effects of docetaxel (Taxotere): a potential radiation sensitizer. Int J Radiat Biol 75: 731–737, doi: 10.1080/095530099140078 - PubMed
-
- Curran WJ, Scott CB, Langer CJ, Komaki R, Lee JS, Hauser S, Movsas B, Wasserman T, Sause W, Cox JD, for the Radiation Therapy Oncology Group (2003) Long-term benefit is observed in a phase III comparison of sequential vs concurrent chemoradiation for patients with unresected stage III nsclc: RTOG 9410. Proc Am Soc Clin Oncol 22: 621 (abstract 2499)
-
- Davies AM, Lara Jr PN, Mack PC, Gandara DR (2003) Docetaxel in non-small cell lung cancer: a review. Expert Opin Pharmacother 4: 553–565 - PubMed
-
- Dillman RO, Herndon J, Seagren SL, Eaton Jr WL, Green MR (1996) Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of Cancer and Leukemia Group B (CALGB) 8433 trial. J Natl Cancer Inst 88: 1210–1215 - PubMed

