Bcl-2 changes conformation to inhibit Bax oligomerization
- PMID: 16642033
- PMCID: PMC1478188
- DOI: 10.1038/sj.emboj.7601126
Bcl-2 changes conformation to inhibit Bax oligomerization
Abstract
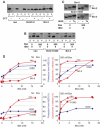
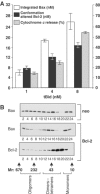
Bcl-2 inhibits apoptosis by regulating the release of cytochrome c and other proteins from mitochondria. Oligomerization of Bax promotes cell death by permeabilizing the outer mitochondrial membrane. In transfected cells and isolated mitochondria, Bcl-2, but not the inactive point mutants Bcl-2-G145A and Bcl-2-V159D, undergoes a conformation change in the mitochondrial membrane in response to apoptotic agonists such as tBid and Bax. A mutant Bcl-2 with two cysteines introduced at positions predicted to result in a disulfide bond that would inhibit the mobility of alpha5-alpha6 helices (Bcl-2-S105C/E152C) was only active in a reducing environment. Thus, Bcl-2 must change the conformation to inhibit tBid-induced oligomerization of integral membrane Bax monomers and small oligomers. The conformationally changed Bcl-2 sequesters the integral membrane form of Bax. If Bax is in excess, apoptosis resumes as Bcl-2 is consumed by the conformational change and in complexes with Bax. Thus, Bcl-2 functions as an inhibitor of mitochondrial permeabilization by changing conformation in the mitochondrial membrane to bind membrane-inserted Bax monomers and prevent productive oligomerization of Bax.
Figures
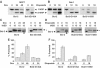

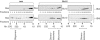


References
-
- Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322–1326 - PubMed
-
- Asoh S, Ohtsu T, Ohta S (2000) The super anti-apoptotic factor Bcl-xFNK constructed by disturbing intramolecular polar interactions in rat Bcl-xL. J Biol Chem 275: 37240–37425 - PubMed
-
- Hsu YT, Youle RJ (1997) Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem 272: 13829–132834 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

