A Drosophila model of oculopharyngeal muscular dystrophy reveals intrinsic toxicity of PABPN1
- PMID: 16642034
- PMCID: PMC1462976
- DOI: 10.1038/sj.emboj.7601117
A Drosophila model of oculopharyngeal muscular dystrophy reveals intrinsic toxicity of PABPN1
Abstract
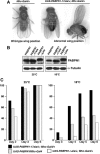
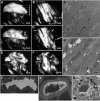
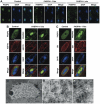
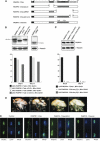
Oculopharyngeal muscular dystrophy (OPMD) is an adult-onset syndrome characterized by progressive degeneration of particular muscles. OPMD is caused by short GCG repeat expansions within the gene encoding the nuclear poly(A)-binding protein 1 (PABPN1) that extend an N-terminal polyalanine tract in the protein. Mutant PABPN1 aggregates as nuclear inclusions in OMPD patient muscles. We have created a Drosophila model of OPMD that recapitulates the features of the human disorder: progressive muscle degeneration, with muscle defects proportional to the number of alanines in the tract, and formation of PABPN1 nuclear inclusions. Strikingly, the polyalanine tract is not absolutely required for muscle degeneration, whereas another domain of PABPN1, the RNA-binding domain and its function in RNA binding are required. This demonstrates that OPMD does not result from polyalanine toxicity, but from an intrinsic property of PABPN1. We also identify several suppressors of the OPMD phenotype. This establishes our OPMD Drosophila model as a powerful in vivo test to understand the disease process and develop novel therapeutic strategies.
Figures

Similar articles
-
Assessment of PABPN1 nuclear inclusions on a large cohort of patients and in a human xenograft model of oculopharyngeal muscular dystrophy.Acta Neuropathol. 2022 Dec;144(6):1157-1170. doi: 10.1007/s00401-022-02503-7. Epub 2022 Oct 5. Acta Neuropathol. 2022. PMID: 36197469 Free PMC article.
-
Novel mouse models of oculopharyngeal muscular dystrophy (OPMD) reveal early onset mitochondrial defects and suggest loss of PABPN1 may contribute to pathology.Hum Mol Genet. 2017 Sep 1;26(17):3235-3252. doi: 10.1093/hmg/ddx206. Hum Mol Genet. 2017. PMID: 28575395 Free PMC article.
-
Mitochondrial dysfunction reveals the role of mRNA poly(A) tail regulation in oculopharyngeal muscular dystrophy pathogenesis.PLoS Genet. 2015 Mar 27;11(3):e1005092. doi: 10.1371/journal.pgen.1005092. eCollection 2015 Mar. PLoS Genet. 2015. PMID: 25816335 Free PMC article.
-
Progress in understanding the pathogenesis of oculopharyngeal muscular dystrophy.Can J Neurol Sci. 2003 Feb;30(1):8-14. doi: 10.1017/s0317167100002365. Can J Neurol Sci. 2003. PMID: 12619777 Review.
-
Oculopharyngeal muscular dystrophy: recent advances in the understanding of the molecular pathogenic mechanisms and treatment strategies.Biochim Biophys Acta. 2007 Feb;1772(2):173-85. doi: 10.1016/j.bbadis.2006.10.003. Epub 2006 Oct 11. Biochim Biophys Acta. 2007. PMID: 17110089 Review.
Cited by
-
Oculopharyngeal muscular dystrophy: a polyalanine myopathy.Curr Neurol Neurosci Rep. 2009 Jan;9(1):76-82. doi: 10.1007/s11910-009-0012-y. Curr Neurol Neurosci Rep. 2009. PMID: 19080757 Review.
-
Interspecies translation of disease networks increases robustness and predictive accuracy.PLoS Comput Biol. 2011 Nov;7(11):e1002258. doi: 10.1371/journal.pcbi.1002258. Epub 2011 Nov 3. PLoS Comput Biol. 2011. PMID: 22072955 Free PMC article.
-
Activation of the ubiquitin-proteasome system contributes to oculopharyngeal muscular dystrophy through muscle atrophy.PLoS Genet. 2022 Jan 13;18(1):e1010015. doi: 10.1371/journal.pgen.1010015. eCollection 2022 Jan. PLoS Genet. 2022. PMID: 35025870 Free PMC article.
-
Similarities of developmental gene expression changes in the brain between human and experimental animals: rhesus monkey, mouse, Zebrafish, and Drosophila.Mol Brain. 2021 Sep 7;14(1):135. doi: 10.1186/s13041-021-00840-4. Mol Brain. 2021. PMID: 34493287 Free PMC article.
-
Protein quality control in the nucleus.Curr Opin Cell Biol. 2016 Jun;40:81-89. doi: 10.1016/j.ceb.2016.03.002. Epub 2016 Mar 22. Curr Opin Cell Biol. 2016. PMID: 27015023 Free PMC article. Review.
References
-
- Abu-Baker A, Messaed C, Laganiere J, Gaspar C, Brais B, Rouleau GA (2003) Involvement of the ubiquitin–proteasome pathway and molecular chaperones in oculopharyngeal muscular dystrophy. Hum Mol Genet 12: 2609–2623 - PubMed
-
- Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S (2004) Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431: 805–810 - PubMed
-
- Bao YP, Cook LJ, O'Donovan D, Uyama E, Rubinsztein DC (2002) Mammalian, yeast, bacterial, and chemical chaperones reduce aggregate formation and death in a cell model of oculopharyngeal muscular dystrophy. J Biol Chem 277: 12263–12269 - PubMed
-
- Bear DG, Fomproix N, Soop T, Bjorkroth B, Masich S, Daneholt B (2003) Nuclear poly(A)-binding protein PABPN1 is associated with RNA polymerase II during transcription and accompanies the released transcript to the nuclear pore. Exp Cell Res 286: 332–344 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

