Potentiometric sensors for trace-level analysis
- PMID: 16642205
- PMCID: PMC1482835
- DOI: 10.1016/j.trac.2005.01.003
Potentiometric sensors for trace-level analysis
Abstract
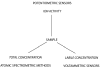
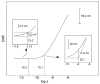
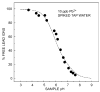
This review summarizes recent progress in the development and application of potentiometric sensors with limits of detection (LODs) in the range 10(-8)-10(-11) M. These LODs relate to total sample concentrations and are defined according to a definition unique to potentiometric sensors. LODs calculated according to traditional protocols (three times the standard deviation of the noise) yield values that are two orders of magnitude lower. We are targeting this article at analytical chemists who are non-specialists in the development of such sensors so that this technology may be adopted by a growing number of research groups to solve real-world analytical problems.We discuss the unique response features of potentiometric sensors and compare them to other analytical techniques, emphasizing that the choice of the method must depend on the problem of interest. We discuss recent directions in sensor design and development and present a list of 23 sensors with low LODs, with references. We give recent examples where potentiometric sensors have been used to solve trace-level analytical problems, including the speciation of lead and copper ions in drinking water, the measurement of free copper in sea water, and the uptake of cadmium ions by plant roots as a function of their speciation.
Figures

Similar articles
-
Potentiometric polymeric membrane electrodes for measurement of environmental samples at trace levels: new requirements for selectivities and measuring protocols, and comparison with ICPMS.Anal Chem. 2001 Jan 15;73(2):343-51. doi: 10.1021/ac001034s. Anal Chem. 2001. PMID: 11199988
-
Reliable environmental trace heavy metal analysis with potentiometric ion sensors - reality or a distant dream.Environ Pollut. 2021 Nov 15;289:117882. doi: 10.1016/j.envpol.2021.117882. Epub 2021 Jul 30. Environ Pollut. 2021. PMID: 34364114 Review.
-
Detection of copper, lead, cadmium and iron in wine using electronic tongue sensor system.Talanta. 2014 Nov;129:63-71. doi: 10.1016/j.talanta.2014.04.030. Epub 2014 Apr 30. Talanta. 2014. PMID: 25127565
-
Development of Chromium(III) Selective Potentiometric Sensors for Its Determination in Petroleum Water Samples Using Synthesized Nano Schiff Base Complex as an Ionophore.J AOAC Int. 2022 Apr 27;105(3):727-738. doi: 10.1093/jaoacint/qsab166. J AOAC Int. 2022. PMID: 34935954
-
Recent Progress on Potentiometric Sensor Applications Based on Nanoscale Metal Oxides: A Comprehensive Review.Crit Rev Anal Chem. 2024 Apr 9:1-18. doi: 10.1080/10408347.2024.2337876. Online ahead of print. Crit Rev Anal Chem. 2024. PMID: 38593048 Review.
Cited by
-
Disposable Multi-Walled Carbon Nanotubes-Based Plasticizer-Free Solid-Contact Pb2+-Selective Electrodes with a Sub-PPB Detection Limit †.Sensors (Basel). 2019 Jun 4;19(11):2550. doi: 10.3390/s19112550. Sensors (Basel). 2019. PMID: 31167473 Free PMC article.
-
Development of Two-Dimensional Functional Nanomaterials for Biosensor Applications: Opportunities, Challenges, and Future Prospects.Nanomaterials (Basel). 2023 Apr 29;13(9):1520. doi: 10.3390/nano13091520. Nanomaterials (Basel). 2023. PMID: 37177065 Free PMC article. Review.
-
Developments in the Field of Conducting and Non-conducting Polymer Based Potentiometric Membrane Sensors for Ions Over the Past Decade.Sensors (Basel). 2008 Apr 3;8(4):2331-2412. doi: 10.3390/s8042331. Sensors (Basel). 2008. PMID: 27879825 Free PMC article. Review.
-
A solid-contact ion selective electrode for copper(II) using a succinimide derivative as ionophore.Sensors (Basel). 2013 Apr 2;13(4):4367-77. doi: 10.3390/s130404367. Sensors (Basel). 2013. PMID: 23549362 Free PMC article.
-
The effect of composition of different ecotoxicological test media on free and bioavailable copper from CuSO4 and CuO nanoparticles: comparative evidence from a Cu-selective electrode and a Cu-biosensor.Sensors (Basel). 2011;11(11):10502-21. doi: 10.3390/s111110502. Epub 2011 Nov 3. Sensors (Basel). 2011. PMID: 22346655 Free PMC article.
References
-
- Buck RP, Lindner E. Anal. Chem. 2001;73:88A. - PubMed
-
- Bakker E, Bühlmann P, Pretsch E. Chem. Rev. 1997;97:3083. - PubMed
-
- Bühlmann P, Pretsch E, Bakker E. Chem. Rev. 1998;98:1593. - PubMed
-
- Bakker E, Pretsch E, Bühlmann P. Anal. Chem. 2000;72:1127. - PubMed
-
- Bakker E, Pretsch E. Trends Anal. Chem. 2001;20:11.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
