Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma
- PMID: 16645163
- PMCID: PMC1895501
- DOI: 10.1182/blood-2006-04-015586
Constitutive activation of Akt contributes to the pathogenesis and survival of mantle cell lymphoma
Abstract
To determine whether the PI3K/Akt signaling pathway is involved in the pathogenesis of mantle cell lymphoma (MCL), we investigated the phosphorylation status of Akt and multiple downstream targets in primary MCL cases and cell lines. Akt was phosphorylated in 12 of 12 aggressive blastoid MCL variants and in 4 of 4 MCL cell lines. In contrast, phosphorylated Akt was present in only 5 of 16 typical MCL, 3 at comparable levels to the blastoid cases, and 2 at low levels. The presence of p-Akt was accompanied by the phosphorylation of p27(kip1), FRKHL-1, MDM2, Bad, mTOR, and p70S6K. Inhibition of the PI3K/Akt pathway in the MCL cell lines abrogated or reduced the phosphorylation of Akt, p27(kip1), FRKHL-1, MDM2, Bad, mTOR, GSK-3beta, IkappaB, and led to cell-cycle arrest and apoptosis. Six MCL cases (5 with activated Akt and 1 with inactive Akt) and 3 of 4 cell lines showed loss of PTEN expression. PIK3CA mutations were not detected. We conclude that constitutive activation of the PI3K/Akt pathway contributes to the pathogenesis of MCL and preferentially occurs in blastoid variants. One possible mechanism of activation is loss of PTEN expression. These data suggest that PI3K/Akt inhibitors may be effective in the treatment of Akt-activated MCL.
Figures





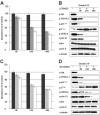
 ), and RAJI (▪). A significant reduction in absorbance to 50% of control values after 48 hours is seen in the MCL cell lines, whereas the non-Akt-activated control RAJI shows no significant reduction. (B,D) Western blot analysis with Granta 519 demonstrates Akt inactivation at 8 hours, followed by abrogation of phosphorylation of FRKHL-1, p27kip1, and GSK-3β by 24 hours. The cell-cycle inhibitor p27kip1 shows a gradual increase in expression levels over time, and cyclin D1 is dramatically down-regulated. In contrast, cdk4 and cyclin E remain constant. α-tubulin expression is shown as loading control.
), and RAJI (▪). A significant reduction in absorbance to 50% of control values after 48 hours is seen in the MCL cell lines, whereas the non-Akt-activated control RAJI shows no significant reduction. (B,D) Western blot analysis with Granta 519 demonstrates Akt inactivation at 8 hours, followed by abrogation of phosphorylation of FRKHL-1, p27kip1, and GSK-3β by 24 hours. The cell-cycle inhibitor p27kip1 shows a gradual increase in expression levels over time, and cyclin D1 is dramatically down-regulated. In contrast, cdk4 and cyclin E remain constant. α-tubulin expression is shown as loading control.
References
-
- Raffeld M, Jaffe ES. bcl-1, t(11;14), and mantle cell derived neoplasms. Blood. 1991;78: 259-263. - PubMed
-
- Meeker TC, Sellers W, Harvey R, et al. Cloning of the t(11;14)(q13;q32) translocation breakpoints from two human leukemia cell lines. Leukemia. 1991;5: 733-737. - PubMed
-
- Tsujimoto Y, Jaffe E, Cossman J, Gorham J, Nowell PC, Croce CM. Clustering of breakpoints on chromosome 11 in human B-cell neoplasms with the t(11;14) chromosome translocation. Nature. 1985;315: 340-343. - PubMed
-
- Bosch F, Lopez-Guillermo A, Campo E, et al. Mantle cell lymphoma: presenting features, response to therapy, and prognostic factors. Cancer. 1998;82: 567-575. - PubMed
-
- Argatoff LH, Connors JM, Klasa RJ, Horsman DE, Gascoyne RD. Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood. 1997;89: 2067-2078. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

