Flanking HS-62.5 and 3' HS1, and regions upstream of the LCR, are not required for beta-globin transcription
- PMID: 16645164
- PMCID: PMC1895883
- DOI: 10.1182/blood-2006-04-014431
Flanking HS-62.5 and 3' HS1, and regions upstream of the LCR, are not required for beta-globin transcription
Abstract
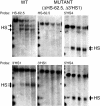
The locus control region (LCR) was thought to be necessary and sufficient for establishing and maintaining an open beta-globin locus chromatin domain in the repressive environment of the developing erythrocyte. However, deletion of the LCR from the endogenous locus had no significant effect on chromatin structure and did not silence transcription. Thus, the cis-regulatory elements that confer the open domain remain unidentified. The conserved DNaseI hypersensitivity sites (HSs) HS-62.5 and 3'HS1 that flank the locus, and the region upstream of the LCR have been implicated in globin gene regulation. The flanking HSs bind CCCTC binding factor (CTCF) and are thought to interact with the LCR to form a "chromatin hub" involved in beta-globin gene activation. Hispanic thalassemia, a deletion of the LCR and 27 kb upstream, leads to heterochromatinization and silencing of the locus. Thus, the region upstream of the LCR deleted in Hispanic thalassemia (upstream Hispanic region [UHR]) may be required for expression. To determine the importance of the UHR and flanking HSs for beta-globin expression, we generated and analyzed mice with targeted deletions of these elements. We demonstrate deletion of these regions alone, and in combination, do not affect transcription, bringing into question current models for the regulation of the beta-globin locus.
Figures

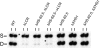
References
-
- Stamatoyannopoulos G, Nienhus AW. Hemoglobin switching. In: Stamatoyannopoulos G, Nienhus AW, Majerus PW, Varmus H, eds. The Molecular Basis of Blood Diseases (2nd ed). Philadelphia, PA: Saunders; 1994: 107-155.
-
- Forrester WC, Epner E, Driscoll MC, et al. A deletion of the human beta-globin locus activation region causes a major alteration in chromatin structure and replication across the entire beta-globin locus. Genes Dev. 1990;4: 1637-1649. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

