Persistent effects induced by IL-13 in the lung
- PMID: 16645178
- PMCID: PMC2643287
- DOI: 10.1165/rcmb.2005-0474OC
Persistent effects induced by IL-13 in the lung
Abstract
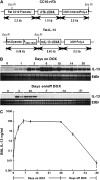
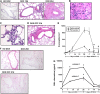
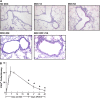
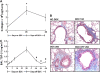
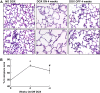
IL-13 overexpression in the lung induces inflammatory and remodeling responses that are prominent features of asthma. Whereas most studies have concentrated on the development of IL-13-induced disease, far fewer studies have focused on the reversibility of IL-13-induced pathologies. This is particularly important because current asthma therapy appears to be poor at reversing lung remodeling. In this manuscript, we used an externally regulatable transgenic system that targets expression of IL-13 to the lung with the aim of characterizing the reversibility process. After 4 wk of doxycycline (dox) exposure, IL-13 expression resulted in mixed inflammatory cell infiltration, mucus cell metaplasia, lung fibrosis, and airspace enlargement (emphysema). After withdrawal of dox, IL-13 protein levels were profoundly reduced by 7 d and below baseline by 14 d. During this time frame, the level of lung eosinophils returned to near normal, whereas macrophages, lymphocytes, and neutrophils remained markedly elevated. IL-13-induced mucus cell metaplasia significantly decreased (91%) 3 wk after withdrawal of dox, showing strong correlation with reduced eosinophil levels. In contrast, IL-13-induced lung fibrosis did not significantly decline 4 wk after dox withdrawal. Importantly, IL-13-induced emphysema persisted, but modestly declined 4 wk after dox. Examination of transcript expression profiles identified a subset of genes that remained increased weeks after transgene expression was no longer detected. Notably, numerous IL-13-induced cytokines and enzymes were reversible (IL-6 and cathepsins), whereas others were sustained (CCL6 and chitinases) after IL-13 withdrawal, respectively. Thus, several hallmark features of IL-13-induced lung pathology persist and are dissociated from eosinophilia after IL-13 overexpression ceases.
Figures

References
-
- Bousquet J, Chanez P, Lacoste JY, White R, Vic P, Godard P, Michel FB. Asthma: a disease remodeling the airways. Allergy 1992;47:3–11. - PubMed
-
- Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258–2261. - PubMed
-
- Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med 2002;8:885–889. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

