Pulmonary immune responses to Propionibacterium acnes in C57BL/6 and BALB/c mice
- PMID: 16645181
- PMCID: PMC2643288
- DOI: 10.1165/rcmb.2005-0285OC
Pulmonary immune responses to Propionibacterium acnes in C57BL/6 and BALB/c mice
Abstract
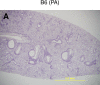
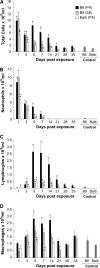
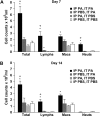
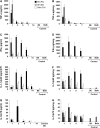
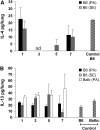
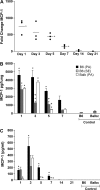
Propionibacterium acnes (PA) is a gram-positive anaerobic bacterium implicated as a putative etiologic agent of sarcoidosis. To characterize the pulmonary immune response to PA, C57BL/6 and BALB/c mice were intraperitoneally sensitized and intratracheally challenged with heat-killed bacteria. C57BL/6 mice challenged with PA developed a cellular immune response characterized by elevations in Th1 cytokines/chemokines, increased numbers of lymphocytes and macrophages in lung lavage fluid, and peribronchovascular granulomatous inflammation composed of T- and B-lymphocytes and epithelioid histiocytes. T-lymphocytes in the lung lavage fluid showed a marked CD4+ cell predominance. In contrast, C57BL/6 mice challenged with Staphylococcus epidermidis (SE), another gram-positive commensal of human skin, and BALB/c mice challenged with PA, showed only a modest induction of Th1 cytokines, less pulmonary inflammation, and no granulomatous changes in the lung. Enhancement of Toll-like receptor expression was seen in PA-exposed C57BL/6 mice within 24 h after exposure, suggesting that induction of innate immunity by PA contributes to the robust, polarized Th1 immune response elicited by this bacterium. These findings suggest that PA-induced pulmonary inflammation may be a useful model for testing the contributions of both bacterial and host factors in the development, maintenance, and resolution of granulomatous inflammation in the lung.
Figures












References
-
- Travis WD, Colby TV, Koss MN, Rosado-de-Christenson ML, Muller NL, King TE. Atlas of non-tumor pathology: non-neoplastic disorders of the lower respiratory tract, 2nd ed. Washington, DC: American Registry of Pathology and the Armed Forces Institute of Pathology; 2002.
-
- Katchar K, Wahlstrom J, Eklund A, Grunewald J. Highly activated T-cell receptor AV2S3(+) CD4(+) lung T-cell expansions in pulmonary sarcoidosis. Am J Respir Crit Care Med 2001;163:1540–1545. - PubMed
-
- Moller DR. Involvement of T cells and alterations in T cell receptors in sarcoidosis. Semin Respir Infect 1998;13:174–183. - PubMed
-
- Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet 2003;361: 1111–1118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

