Cyclooxygenases and prostaglandin E2 receptors in growth plate chondrocytes in vitro and in situ--prostaglandin E2 dependent proliferation of growth plate chondrocytes
- PMID: 16646980
- PMCID: PMC1526634
- DOI: 10.1186/ar1948
Cyclooxygenases and prostaglandin E2 receptors in growth plate chondrocytes in vitro and in situ--prostaglandin E2 dependent proliferation of growth plate chondrocytes
Abstract
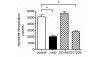
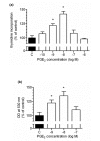
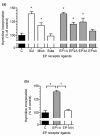
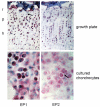
Prostaglandin E2 (PGE2) plays an important role in bone development and metabolism. To interfere therapeutically in the PGE2 pathway, however, knowledge about the involved enzymes (cyclooxygenases) and receptors (PGE2 receptors) is essential. We therefore examined the production of PGE2 in cultured growth plate chondrocytes in vitro and the effects of exogenously added PGE2 on cell proliferation. Furthermore, we analysed the expression and spatial distribution of cyclooxygenase (COX)-1 and COX-2 and PGE2 receptor types EP1, EP2, EP3 and EP4 in the growth plate in situ and in vitro. PGE2 synthesis was determined by mass spectrometry, cell proliferation by DNA [3H]-thymidine incorporation, mRNA expression of cyclooxygenases and EP receptors by RT-PCR on cultured cells and in homogenized growth plates. To determine cellular expression, frozen sections of rat tibial growth plate and primary chondrocyte cultures were stained using immunohistochemistry with polyclonal antibodies directed towards COX-1, COX-2, EP1, EP2, EP3, and EP4. Cultured growth plate chondrocytes transiently secreted PGE2 into the culture medium. Although both enzymes were expressed in chondrocytes in vitro and in vivo, it appears that mainly COX-2 contributed to PGE2-dependent proliferation. Exogenously added PGE2 stimulated DNA synthesis in a dose-dependent fashion and gave a bell-shaped curve with a maximum at 10-8 M. The EP1/EP3 specific agonist sulprostone and the EP1-selective agonist ONO-D1-004 increased DNA synthesis. The effect of PGE2 was suppressed by ONO-8711. The expression of EP1, EP2, EP3, and EP4 receptors in situ and in vitro was observed; EP2 was homogenously expressed in all zones of the growth plate in situ, whereas EP1 expression was inhomogenous, with spared cells in the reserve zone. In cultured cells these four receptors were expressed in a subset of cells only. The most intense staining for the EP1 receptor was found in polygonal cells surrounded by matrix. Expression of receptor protein for EP3 and EP4 was observed also in rat growth plates. In cultured chrondrocytes, however, only weak expression of EP3 and EP4 receptor was detected. We suggest that in growth plate chondrocytes, COX-2 is responsible for PGE2 release, which stimulates cell proliferation via the EP1 receptor.
Figures





Similar articles
-
Characterization of PGE(2) receptors (EP) and their role as mediators of 1alpha,25-(OH)(2)D(3) effects on growth zone chondrocytes.J Steroid Biochem Mol Biol. 2001 Sep;78(3):261-74. doi: 10.1016/s0960-0760(01)00099-1. J Steroid Biochem Mol Biol. 2001. PMID: 11595507
-
Cyclooxygenase-2-dependent prostaglandin E2 down-regulates intercellular adhesion molecule-1 expression via EP2/EP4 receptors in interleukin-1beta-stimulated human gingival fibroblasts.J Dent Res. 2000 Dec;79(12):1955-61. doi: 10.1177/00220345000790120601. J Dent Res. 2000. PMID: 11201045
-
Role of prostaglandin E2 receptors in migration of murine and human breast cancer cells.Exp Cell Res. 2003 Oct 1;289(2):265-74. doi: 10.1016/s0014-4827(03)00269-6. Exp Cell Res. 2003. PMID: 14499627
-
Roles of Cyclooxygenase, Prostaglandin E2 and EP Receptors in Mucosal Protection and Ulcer Healing in the Gastrointestinal Tract.Curr Pharm Des. 2018;24(18):2002-2011. doi: 10.2174/1381612824666180629111227. Curr Pharm Des. 2018. PMID: 29956615 Review.
-
[Cooperation of two subtypes of PGE2 receptor, Gi coupled EP3 and Gs coupled EP2 or EP4 subtype].Yakugaku Zasshi. 2003 Oct;123(10):837-43. doi: 10.1248/yakushi.123.837. Yakugaku Zasshi. 2003. PMID: 14577329 Review. Japanese.
Cited by
-
[Tissue engineering of cartilage and bone : growth factors and signaling molecules].Orthopade. 2009 Nov;38(11):1053-62. doi: 10.1007/s00132-009-1496-5. Orthopade. 2009. PMID: 19851750 German.
-
Inhibition of the Prostaglandin EP-1 Receptor in Periosteum Progenitor Cells Enhances Osteoblast Differentiation and Fracture Repair.Ann Biomed Eng. 2020 Mar;48(3):927-939. doi: 10.1007/s10439-019-02264-7. Epub 2019 Apr 12. Ann Biomed Eng. 2020. PMID: 30980293 Free PMC article.
-
Stability of prostaglandin E(2) (PGE (2)) embedded in poly-D,L: -lactide-co-glycolide microspheres: a pre-conditioning approach for tissue engineering applications.J Mater Sci Mater Med. 2009 Jun;20(6):1357-65. doi: 10.1007/s10856-008-3678-9. Epub 2009 Jan 22. J Mater Sci Mater Med. 2009. PMID: 19160024
-
The Essentiality of Arachidonic Acid in Infant Development.Nutrients. 2016 Apr 12;8(4):216. doi: 10.3390/nu8040216. Nutrients. 2016. PMID: 27077882 Free PMC article. Review.
-
Prostacyclin regulates bone growth via the Epac/Rap1 pathway.Endocrinology. 2015 Feb;156(2):499-510. doi: 10.1210/en.2014-1348. Epub 2014 Nov 18. Endocrinology. 2015. PMID: 25406016 Free PMC article.
References
-
- Klein DC, Raisz LG. Prostaglandins: stimulation of bone resorption in tissue culture. Endocrinology. 1970;86:1436–1440. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

