Folate-targeted immunotherapy effectively treats established adjuvant and collagen-induced arthritis
- PMID: 16646988
- PMCID: PMC1526647
- DOI: 10.1186/ar1944
Folate-targeted immunotherapy effectively treats established adjuvant and collagen-induced arthritis
Abstract
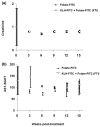
Activated macrophages express a cell surface receptor for the vitamin folic acid. Because this receptor is inaccessible or not measurably expressed on other normal cells, folic acid has been recently exploited to selectively deliver attached radio-emitters to sites of activated macrophage accumulation, allowing scintigraphic imaging of inflamed joints and organs of arthritic rats. We demonstrate here that folate-linked haptens can also be targeted to activated macrophages, decorating their cell surfaces with highly immunogenic molecules. Under conditions in which the rodent has already been immunized against keyhole limpet hemocyanine-(fluorescein isothiocyanate) FITC, activated macrophages are eliminated. Administration of folate-FITC conjugates to rodents with experimental arthritis attenuates (a) systemic and peri-articular inflammation, (b) bone and cartilage degradation, and (c) arthritis-related body weight loss. Treatment with folate-hapten conjugates is comparable to methotrexate, etanercept, anakinra, and celecoxib at alleviating the symptoms of arthritis. We conclude that reduction of activated macrophages by folate-targeted immunotherapy can ameliorate the symptoms of arthritis in two rodent models of the disease.
Figures

Similar articles
-
Folate-targeted hapten immunotherapy of adjuvant-induced arthritis: comparison of hapten potencies.Mol Pharm. 2009 Jul-Aug;6(4):1228-36. doi: 10.1021/mp900070b. Mol Pharm. 2009. PMID: 19374407
-
Optimization of Folate-Targeted Immunotherapy for the Treatment of Experimental Arthritis.Inflammation. 2016 Aug;39(4):1345-53. doi: 10.1007/s10753-016-0366-7. Inflammation. 2016. PMID: 27206918
-
Folate-targeted imaging of activated macrophages in rats with adjuvant-induced arthritis.Arthritis Rheum. 2002 Jul;46(7):1947-55. doi: 10.1002/art.10405. Arthritis Rheum. 2002. PMID: 12124880
-
Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis.Adv Drug Deliv Rev. 2004 Apr 29;56(8):1205-17. doi: 10.1016/j.addr.2004.01.012. Adv Drug Deliv Rev. 2004. PMID: 15094216 Review.
-
Folate receptor-targeted immunotherapy of cancer: mechanism and therapeutic potential.Adv Drug Deliv Rev. 2004 Apr 29;56(8):1161-76. doi: 10.1016/j.addr.2004.01.009. Adv Drug Deliv Rev. 2004. PMID: 15094213 Review.
Cited by
-
Folate Receptor-Targeting and Reactive Oxygen Species-Responsive Liposomal Formulation of Methotrexate for Treatment of Rheumatoid Arthritis.Pharmaceutics. 2019 Nov 6;11(11):582. doi: 10.3390/pharmaceutics11110582. Pharmaceutics. 2019. PMID: 31698794 Free PMC article.
-
Low-dose methotrexate inhibits methionine S-adenosyltransferase in vitro and in vivo.Mol Med. 2012 May 9;18(1):423-32. doi: 10.2119/molmed.2011.00048. Mol Med. 2012. PMID: 22193356 Free PMC article.
-
Depletion of activated macrophages with a folate receptor-beta-specific antibody improves symptoms in mouse models of rheumatoid arthritis.Arthritis Res Ther. 2019 Jun 7;21(1):143. doi: 10.1186/s13075-019-1912-0. Arthritis Res Ther. 2019. PMID: 31174578 Free PMC article.
-
Dapagliflozin targets the crosstalk between apoptosis, autophagy, and Hedgehog signaling pathways through AMPK activation in the adjuvant-induced arthritic rat model.Inflammopharmacology. 2025 Jun;33(6):3157-3176. doi: 10.1007/s10787-025-01750-w. Epub 2025 May 12. Inflammopharmacology. 2025. PMID: 40350466 Free PMC article.
-
Antiinflammatory Activity of a Novel Folic Acid Targeted Conjugate of the mTOR Inhibitor Everolimus.Mol Med. 2015 Jul 8;21(1):584-96. doi: 10.2119/molmed.2015.00040. Mol Med. 2015. PMID: 26181632 Free PMC article.
References
-
- Tak PP, Smeets TJ, Daha MR, Kluin PM, Meijers KA, Brand R, Meinders AE, Breedveld FC. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis Rheum. 1997;40:217–225. - PubMed
-
- Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39:115–124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

