Modeling Hsp70-mediated protein folding
- PMID: 16648174
- PMCID: PMC1483108
- DOI: 10.1529/biophysj.106.083394
Modeling Hsp70-mediated protein folding
Abstract
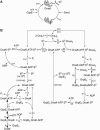
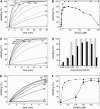
The Hsp70 chaperone system is the major molecular chaperone system that assists protein-folding processes in all cells. To understand these processes, we analyzed the kinetic characteristics of the Escherichia coli homologs of this chaperone system during folding of a denatured protein using computer simulations and compared the results with in vitro refolding experiments. Rate constants used for the model were derived from recent literature or were determined and scrutinized for their applicability to the refolding reaction. Our simulation results are consistent with reported laboratory experiments, not only simulating the refolding reaction of wild-type proteins but also the behavior of mutant variants. Variation of kinetic parameters and concentrations of components of the Hsp70 system demonstrate the robustness of the chaperone system in assisting protein folding. Furthermore, the importance of the synergistic stimulation of the ATPase activity of Hsp70 is demonstrated. The limitations of our kinetic model indicate sore spots in our understanding of this chaperone system. Our model provides a platform for further research on chaperone action and the mechanism of chaperone-assisted refolding of denatured proteins.
Figures




Similar articles
-
Directed evolution of the DnaK chaperone: mutations in the lid domain result in enhanced chaperone activity.J Mol Biol. 2010 May 28;399(1):154-67. doi: 10.1016/j.jmb.2010.03.060. Epub 2010 Apr 8. J Mol Biol. 2010. PMID: 20381501
-
The unfolding story of the Escherichia coli Hsp70 DnaK: is DnaK a holdase or an unfoldase?Mol Microbiol. 2002 Sep;45(5):1197-206. doi: 10.1046/j.1365-2958.2002.03093.x. Mol Microbiol. 2002. PMID: 12207689 Review.
-
The heat-sensitive Escherichia coli grpE280 phenotype: impaired interaction of GrpE(G122D) with DnaK.J Mol Biol. 2005 Nov 4;353(4):888-96. doi: 10.1016/j.jmb.2005.08.069. Epub 2005 Sep 20. J Mol Biol. 2005. PMID: 16198374
-
[Mutation clpA::kan in gene encoding the chaperone of Hsp100-family decreases DnaK-dependent refolding efficiency of proteins in Escherichia coli cells].Mol Biol (Mosk). 2008 Nov-Dec;42(6):1018-22. Mol Biol (Mosk). 2008. PMID: 19140322 Russian.
-
Hsp90 and Hsp70 chaperones: Collaborators in protein remodeling.J Biol Chem. 2019 Feb 8;294(6):2109-2120. doi: 10.1074/jbc.REV118.002806. Epub 2018 Nov 6. J Biol Chem. 2019. PMID: 30401745 Free PMC article. Review.
Cited by
-
A pharmacological investigation of Hippophae salicifolia (HS) and Hippophae rhamnoides turkestanica (HRT) against multiple stress (C-H-R): an experimental study using rat model.Cell Stress Chaperones. 2015 Sep;20(5):821-31. doi: 10.1007/s12192-015-0603-2. Epub 2015 Jun 5. Cell Stress Chaperones. 2015. PMID: 26045201 Free PMC article.
-
Protein folding rates and thermodynamic stability are key determinants for interaction with the Hsp70 chaperone system.Protein Sci. 2012 Oct;21(10):1489-502. doi: 10.1002/pro.2139. Protein Sci. 2012. PMID: 22886941 Free PMC article.
-
The Hsp70 chaperone system maintains high concentrations of active proteins and suppresses ATP consumption during heat shock.Syst Synth Biol. 2007 Mar;1(1):47-58. doi: 10.1007/s11693-006-9004-2. Epub 2007 Jan 26. Syst Synth Biol. 2007. PMID: 19003436 Free PMC article.
-
Spatial localisation of chaperone distribution in the endoplasmic reticulum of yeast.IET Syst Biol. 2012 Apr;6(2):54-63. doi: 10.1049/iet-syb.2011.0006. IET Syst Biol. 2012. PMID: 22519358 Free PMC article.
-
FoldEco: a model for proteostasis in E. coli.Cell Rep. 2012 Mar 29;1(3):265-76. doi: 10.1016/j.celrep.2012.02.011. Cell Rep. 2012. PMID: 22509487 Free PMC article.
References
-
- Gething, M. J. 1991. Molecular chaperones: individualists or groupies? Curr. Opin. Cell Biol. 3:610–614. - PubMed
-
- Tomoyasu, T., A. Mogk, H. Langen, P. Goloubinoff, and B. Bukau. 2001. Genetic dissection of the roles of chaperones and proteases in protein folding and degradation in the Escherichia cytosol. Mol. Microbiol. 40:397–413. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

