Dimerization and interactions of Brucella suis VirB8 with VirB4 and VirB10 are required for its biological activity
- PMID: 16648257
- PMCID: PMC1464329
- DOI: 10.1073/pnas.0600862103
Dimerization and interactions of Brucella suis VirB8 with VirB4 and VirB10 are required for its biological activity
Abstract
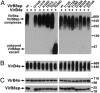
VirB8-like proteins are essential components of type IV secretion systems, bacterial virulence factors that mediate the translocation of effector molecules from many bacterial pathogens into eukaryotic cells. Based on cell biological, genetic, and x-ray crystallographic data, VirB8 was proposed to undergo multiple protein-protein interactions to mediate assembly of the translocation machinery. Here we report the results of a structure-function analysis of the periplasmic domain of VirB8 from the mammalian pathogen Brucella suis, which identifies amino acid residues required for three protein-protein interactions. VirB8 variants changed at residues proposed to be involved in dimerization, and protein-protein interactions were purified and characterized in vitro and in vivo. Changes at M102, Y105, and E214 affected the self-association as measured by analytical ultracentrifugation and gel filtration. The interaction with B. suis VirB10 was reduced by changes at T201, and change at R230 inhibited the interaction with VirB4 in vitro. The in vivo functionality of VirB8 variants was determined by complementation of growth in macrophages by a B. suis virB8 mutant and by using a heterologous assay of type IV secretion system assembly in Agrobacterium tumefaciens. Changes at Y105, T201, R230, and at several other residues impaired the in vivo function of VirB8, suggesting that we have identified interaction sites of relevance in the natural biological context.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



Similar articles
-
Interactions between Brucella suis VirB8 and its homolog TraJ from the plasmid pSB102 underline the dynamic nature of type IV secretion systems.J Bacteriol. 2009 May;191(9):2985-92. doi: 10.1128/JB.01426-08. Epub 2009 Feb 27. J Bacteriol. 2009. PMID: 19251859 Free PMC article.
-
Structural Analysis and Inhibition of TraE from the pKM101 Type IV Secretion System.J Biol Chem. 2016 Nov 4;291(45):23817-23829. doi: 10.1074/jbc.M116.753327. Epub 2016 Sep 15. J Biol Chem. 2016. PMID: 27634044 Free PMC article.
-
Quantitative analysis of VirB8-VirB9-VirB10 interactions provides a dynamic model of type IV secretion system core complex assembly.Biochemistry. 2010 Jun 1;49(21):4483-93. doi: 10.1021/bi902201y. Biochemistry. 2010. PMID: 20426418
-
A single amino acid change in the transmembrane domain of the VirB8 protein affects dimerization, interaction with VirB10 and Brucella suis virulence.FEBS Lett. 2011 Aug 4;585(15):2431-6. doi: 10.1016/j.febslet.2011.07.004. Epub 2011 Jul 13. FEBS Lett. 2011. PMID: 21763312
-
VirB8: a conserved type IV secretion system assembly factor and drug target.Biochem Cell Biol. 2006 Dec;84(6):890-9. doi: 10.1139/o06-148. Biochem Cell Biol. 2006. PMID: 17215876 Review.
Cited by
-
VirB3 to VirB6 and VirB8 to VirB11, but not VirB7, are essential for mediating persistence of Brucella in the reticuloendothelial system.J Bacteriol. 2008 Jul;190(13):4427-36. doi: 10.1128/JB.00406-08. Epub 2008 May 9. J Bacteriol. 2008. PMID: 18469100 Free PMC article.
-
Bacteria-Killing Type IV Secretion Systems.Front Microbiol. 2019 May 21;10:1078. doi: 10.3389/fmicb.2019.01078. eCollection 2019. Front Microbiol. 2019. PMID: 31164878 Free PMC article. Review.
-
The small heat-shock protein HspL is a VirB8 chaperone promoting type IV secretion-mediated DNA transfer.J Biol Chem. 2010 Jun 25;285(26):19757-66. doi: 10.1074/jbc.M110.110296. Epub 2010 Apr 28. J Biol Chem. 2010. PMID: 20427270 Free PMC article.
-
VirB8 homolog TraE from plasmid pKM101 forms a hexameric ring structure and interacts with the VirB6 homolog TraD.Proc Natl Acad Sci U S A. 2018 Jun 5;115(23):5950-5955. doi: 10.1073/pnas.1802501115. Epub 2018 May 21. Proc Natl Acad Sci U S A. 2018. PMID: 29784815 Free PMC article.
-
Structural Insight into How Bacteria Prevent Interference between Multiple Divergent Type IV Secretion Systems.mBio. 2015 Dec 8;6(6):e01867-15. doi: 10.1128/mBio.01867-15. mBio. 2015. PMID: 26646013 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

