Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation
- PMID: 16648270
- PMCID: PMC1564282
- DOI: 10.1073/pnas.0503465103
Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation
Abstract
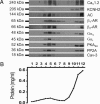
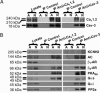
L-type Ca(2+) channels play a critical role in regulating Ca(2+)-dependent signaling in cardiac myocytes, including excitation-contraction coupling; however, the subcellular localization of cardiac L-type Ca(2+) channels and their regulation are incompletely understood. Caveolae are specialized microdomains of the plasmalemma rich in signaling molecules and supported by the structural protein caveolin-3 in muscle. Here we demonstrate that a subpopulation of L-type Ca(2+) channels is localized to caveolae in ventricular myocytes as part of a macromolecular signaling complex necessary for beta(2)-adrenergic receptor (AR) regulation of I(Ca,L). Immunofluorescence studies of isolated ventricular myocytes using confocal microscopy detected extensive colocalization of caveolin-3 and the major pore-forming subunit of the L-type Ca channel (Ca(v)1.2). Immunogold electron microscopy revealed that these proteins colocalize in caveolae. Immunoprecipitation from ventricular myocytes using anti-Ca(v)1.2 or anti-caveolin-3 followed by Western blot analysis showed that caveolin-3, Ca(v)1.2, beta(2)-AR (not beta(1)-AR), G protein alpha(s), adenylyl cyclase, protein kinase A, and protein phosphatase 2a are closely associated. To determine the functional impact of the caveolar-localized beta(2)-AR/Ca(v)1.2 signaling complex, beta(2)-AR stimulation (salbutamol plus atenolol) of I(Ca,L) was examined in pertussis toxin-treated neonatal mouse ventricular myocytes. The stimulation of I(Ca,L) in response to beta(2)-AR activation was eliminated by disruption of caveolae with 10 mM methyl beta-cyclodextrin or by small interfering RNA directed against caveolin-3, whereas beta(1)-AR stimulation (norepinephrine plus prazosin) of I(Ca,L) was not altered. These findings demonstrate that subcellular localization of L-type Ca(2+) channels to caveolar macromolecular signaling complexes is essential for regulation of the channels by specific signaling pathways.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

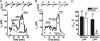
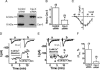
Comment in
-
The real estate of cardiac signaling: location, location, location.Proc Natl Acad Sci U S A. 2006 May 16;103(20):7535-6. doi: 10.1073/pnas.0602389103. Epub 2006 May 8. Proc Natl Acad Sci U S A. 2006. PMID: 16682624 Free PMC article. No abstract available.
References
-
- Balijepalli R. C., Lokuta A. J., Maertz N. A., Buck J. M., Haworth R. A., Valdivia H. H., Kamp T. J. Cardiovasc. Res. 2003;59:67–77. - PubMed
-
- Franzini-Armstrong C., Protasi F., Ramesh V. Ann. N.Y. Acad. Sci. 1998;853:20–30. - PubMed
-
- Tang Z., Scherer P. E., Okamoto T., Song K., Chu C., Kohtz D. S., Nishimoto I., Lodish H. F., Lisanti M. P. J. Biol. Chem. 1996;271:2255–2261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

