Quantitative analysis and subcellular distribution of mRNA and protein expression of the hyperpolarization-activated cyclic nucleotide-gated channels throughout development in rat hippocampus
- PMID: 16648453
- PMCID: PMC3100721
- DOI: 10.1093/cercor/bhk021
Quantitative analysis and subcellular distribution of mRNA and protein expression of the hyperpolarization-activated cyclic nucleotide-gated channels throughout development in rat hippocampus
Abstract
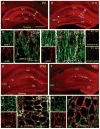
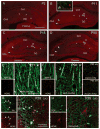
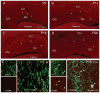
The properties of the hyperpolarization-activated current (I(h)) and its roles in hippocampal network function evolve radically during development. Because I(h) is conducted by the hyperpolarization-activated cyclic nucleotide-gated (HCN) cation channels, we tested the hypothesis that understanding the quantitative developmental profiles of HCN1, HCN2, and HCN4 expression, and the isoform- and age-specific progression of their subcellular distribution, should shed light on the established modifications of the properties of I(h) throughout development. Combined quantitative in situ hybridization, regional western blots, and high-resolution, dual-label immunocytochemistry revealed striking and novel information about the expression and distribution of the HCN channel isoforms in the developing hippocampal formation. In cornus ammon 1 (CA) pyramidal cell layer, a robust increase of HCN1 mRNA and protein expression occurred with age, with reciprocal reduction of HCN4 and relatively stable HCN2 levels. These distinct expression patterns raised the contribution of HCN1 to the total HCN channel pool from 33% to 65% consonant with acceleration and reduced cyclic adenosine mono phosphate (cAMP) sensitivity of I(h) in this region with age. In CA3, strong expression of HCN1 already neonatally supports the recently established role of this conductance in neonatal, age-specific, hippocampal oscillations (giant depolarizing potentials). Notably, HCN1 channels were present and probably transported to dendritic compartments already on postnatal day (P) 2, whereas HCN2 channel protein was not evident in dendrites for the first 2 weeks of life. HCN2 mRNA and protein expression remained fairly constant subsequent to the first week of life in all hippocampal subfields examined, whereas HCN4 mRNA and protein expression declined after maximal neonatal expression, so that the contribution of this isoform to the total HCN channel pool dropped from 43% (CA1) and 34% (CA3) on P11 to 8% (CA1) and 19% (CA3) on P90. Interneuronal expression of all HCN channel isoforms in stratum pyramidale was robust in parvalbumin-but not in cholecystokinin-expressing populations and with a subunit-specific subcellular distribution. Taken together, these data suggest that early in life, HCN4 may contribute significantly to the functions of I(h) in specific hippocampal regions. In addition, these evolving, differential quantitative, and subcellular expression patterns of the HCN channel isoforms support age-specific properties and functions of I(h) within the developing hippocampal formation.
Conflict of interest statement
Figures

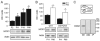

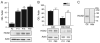

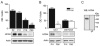
References
-
- Beaumont V, Zucker RS. Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nat Neurosci. 2000;3:133–141. - PubMed
-
- Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24:353–360. - PubMed
-
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. - PubMed
-
- Bender RA, Brewster AL, Santoro B, Ludwig A, Hofmann F, Biel M, Baram TZ. Differential and age-dependent expression of hyperpolarization-activated, cyclic nucleotide-gated cation channel isoforms 1-4 suggests evolving roles in the developing rat hippocampus. Neuroscience. 2001;106:689–698. - PMC - PubMed

