Regulation of cardiac stress signaling by protein kinase d1
- PMID: 16648482
- PMCID: PMC1488991
- DOI: 10.1128/MCB.26.10.3875-3888.2006
Regulation of cardiac stress signaling by protein kinase d1
Abstract
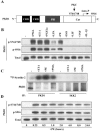
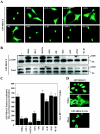
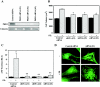
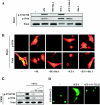
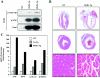
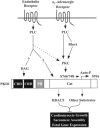
In response to pathological stresses such as hypertension or myocardial infarction, the heart undergoes a remodeling process that is associated with myocyte hypertrophy, myocyte death, and fibrosis. Histone deacetylase 5 (HDAC5) is a transcriptional repressor of cardiac remodeling that is subject to phosphorylation-dependent neutralization in response to stress signaling. Recent studies have suggested a role for protein kinase C (PKC) and its downstream effector, protein kinase D1 (PKD1), in the control of HDAC5 phosphorylation. While PKCs are well-documented regulators of cardiac signaling, the function of PKD1 in heart muscle remains unclear. Here, we demonstrate that PKD1 catalytic activity is stimulated in cardiac myocytes by diverse hypertrophic agonists that signal through G protein-coupled receptors (GPCRs) and Rho GTPases. PKD1 activation in cardiomyocytes occurs through PKC-dependent and -independent mechanisms. In vivo, cardiac PKD1 is activated in multiple rodent models of pathological cardiac remodeling. PKD1 activation correlates with phosphorylation-dependent nuclear export of HDAC5, and reduction of endogenous PKD1 expression with small interfering RNA suppresses HDAC5 shuttling and associated cardiomyocyte growth. Conversely, ectopic overexpression of constitutively active PKD1 in mouse heart leads to dilated cardiomyopathy. These findings support a role for PKD1 in the control of pathological remodeling of the heart via its ability to phosphorylate and neutralize HDAC5.
Figures



References
-
- Antos, C. L., T. A. McKinsey, M. Dreitz, L. M. Hollingsworth, C. L. Zhang, K. Schreiber, H. Rindt, R. J. Gorczynski, and E. N. Olson. 2003. Dose-dependent blockade to cardiomyocyte hypertrophy by histone deacetylase inhibitors. J. Biol. Chem. 278:28930-28937. - PubMed
-
- Auer, A., B. J. von Blume, S. Sturany, W. G. von Wichert, L. J. Van Lint, J. Vandenheede, G. Adler, and T. Seufferlein. 2005. Role of the regulatory domain of protein kinase D2 in phorbol ester binding, catalytic activity, and nucleocytoplasmic shuttling. Mol. Biol. Cell 16:4375-4385. - PMC - PubMed
-
- Bristow, M. R., N. E. Kantrowitz, R. Ginsburg, and M. B. Fowler. 1985. Beta-adrenergic function in heart muscle disease and heart failure. J. Mol. Cell Cardiol. 17(Suppl. 2):41-52. - PubMed
-
- Carnegie, G. K., F. D. Smith, G. McConnachie, L. K. Langeberg, and J. D. Scott. 2004. AKAP-Lbc nucleates a protein kinase D activation scaffold. Mol. Cell 15:889-899. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
