Early embryonic lethality due to targeted inactivation of DNA ligase III
- PMID: 16648486
- PMCID: PMC1489003
- DOI: 10.1128/MCB.26.10.3935-3941.2006
Early embryonic lethality due to targeted inactivation of DNA ligase III
Abstract
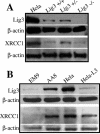
DNA ligases catalyze the joining of strand breaks in the phosphodiester backbone of duplex DNA and play essential roles in DNA replication, recombination, repair, and maintenance of genomic integrity. Three mammalian DNA ligase genes have been identified, and their corresponding ligases play distinct roles in DNA metabolism. DNA ligase III is proposed to be involved in the repairing of DNA single-strand breaks, but its precise role has not yet been demonstrated directly. To determine its role in DNA repair, cellular growth, and embryonic development, we introduced targeted interruption of the DNA ligase III (LIG3) gene into the mouse. Mice homozygous for LIG3 disruption showed early embryonic lethality. We found that the mutant embryonic developmental process stops at 8.5 days postcoitum (dpc), and excessive cell death occurs at 9.5 dpc. LIG3 mutant cells have relatively normal XRCC1 levels but elevated sister chromatid exchange. These findings indicate that DNA ligase III is involved in essential DNA repair activities required for early embryonic development and therefore cannot be replaced by other DNA ligases.
Figures



References
-
- Barnes, D. E., G. Stamp, I. Rosewell, A. Denzel, and T. Lindahl. 1998. Targeted disruption of the gene encoding DNA ligase IV leads to lethality in embryonic mice. Curr. Biol. 8:1395-1398. - PubMed
-
- Caldecott, K. W. 2001. Mammalian DNA single-strand break repair: an X-ra(y)ted affair. Bioessays 23:447-455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
