Functional interaction of CP2 with GATA-1 in the regulation of erythroid promoters
- PMID: 16648487
- PMCID: PMC1489008
- DOI: 10.1128/MCB.26.10.3942-3954.2006
Functional interaction of CP2 with GATA-1 in the regulation of erythroid promoters
Abstract
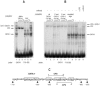
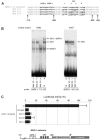
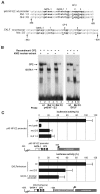
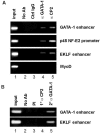
We observed that binding sites for the ubiquitously expressed transcription factor CP2 were present in regulatory regions of multiple erythroid genes. In these regions, the CP2 binding site was adjacent to a site for the erythroid factor GATA-1. Using three such regulatory regions (from genes encoding the transcription factors GATA-1, EKLF, and p45 NF-E2), we demonstrated the functional importance of the adjacent CP2/GATA-1 sites. In particular, CP2 binds to the GATA-1 HS2 enhancer, generating a ternary complex with GATA-1 and DNA. Mutations in the CP2 consensus greatly impaired HS2 activity in transient transfection assays with K562 cells. Similar results were obtained by transfection of EKLF and p45 NF-E2 mutant constructs. Chromatin immunoprecipitation with K562 cells showed that CP2 binds in vivo to all three regulatory elements and that both GATA-1 and CP2 were present on the same GATA-1 and EKLF regulatory elements. Adjacent CP2/GATA-1 sites may represent a novel module for erythroid expression of a number of genes. Additionally, coimmunoprecipitation and glutathione S-transferase pull-down experiments demonstrated a physical interaction between GATA-1 and CP2. This may contribute to the functional cooperation between these factors and provide an explanation for the important role of ubiquitous CP2 in the regulation of erythroid genes.
Figures
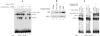
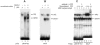

References
-
- Anderson, K. P., S. C. Crable, and J. B. Lingrel. 1998. Multiple proteins binding to a GATA-E box-GATA motif regulate the erythroid Kruppel-like factor (EKLF) gene. J. Biol. Chem. 273:14347-14354. - PubMed
-
- Anderson, K. P., S. C. Crable, and J. B. Lingrel. 2000. The GATA-E box-GATA motif in the EKLF promoter is required for in vivo expression. Blood 95:1652-1655. - PubMed
-
- Bekri, S., A. May, P. D. Cotter, A. I. Al Sabah, X. Guo, G. S. Masters, and D. F. Bishop. 2003. A promoter mutation in the erythroid-specific 5-aminolevulinate synthase (ALAS2) gene causes X-linked sideroblastic anemia. Blood 102:698-704. - PubMed
-
- Cairns, L., M. Ciro, M. Minuzzo, F. Morle, J. Starck, S. Ottolenghi, and A. Ronchi. 2003. Induction of globin mRNA expression by interleukin-3 in a stem cell factor-dependent SV-40 T-antigen-immortalized multipotent hematopoietic cell line. J. Cell. Physiol. 195:38-49. - PubMed
-
- Cantor, A. B., and S. H. Orkin. 2001. Hematopoietic development: a balancing act. Curr. Opin. Genet. Dev. 11:513-519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
