Epidermal growth factor receptor and transforming growth factor-beta signaling contributes to variation for wing shape in Drosophila melanogaster
- PMID: 16648592
- PMCID: PMC1526698
- DOI: 10.1534/genetics.105.053868
Epidermal growth factor receptor and transforming growth factor-beta signaling contributes to variation for wing shape in Drosophila melanogaster
Abstract
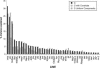
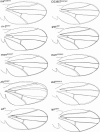
Wing development in Drosophila is a common model system for the dissection of genetic networks and their roles during development. In particular, the RTK and TGF-beta regulatory networks appear to be involved with numerous aspects of wing development, including patterning, cell determination, growth, proliferation, and survival in the developing imaginal wing disc. However, little is known as to how subtle changes in the function of these genes may contribute to quantitative variation for wing shape, per se. In this study 50 insertional mutations, representing 43 loci in the RTK, Hedgehog, TGF-beta pathways, and their genetically interacting factors were used to study the role of these networks on wing shape. To concurrently examine how genetic background modulates the effects of the mutation, each insertion was introgressed into two wild-type genetic backgrounds. Using geometric morphometric methods, it is shown that the majority of these mutations have profound effects on shape but not size of the wing when measured as heterozygotes. To examine the relationships between how each mutation affects wing shape hierarchical clustering was used. Unlike previous observations of environmental canalization, these mutations did not generally increase within-line variation relative to their wild-type counterparts. These results provide an entry point into the genetics of wing shape and are discussed within the framework of the dissection of complex phenotypes.
Figures



References
-
- Atallah, J., I. Dworkin, U. Cheung, A. Greene, B. Ing et al., 2004. The environmental and genetic regulation of obake expressivity: morphogenetic fields as evolvable systems. Evol. Dev. 6: 114–122. - PubMed
-
- Baena-Lopez, L. A., A. Baonza and A. Garcia-Bellido, 2005. The orientation of cell divisions determines the shape of Drosophila organs. Curr. Biol. 15: 1640–1644. - PubMed
-
- Birdsall, K., E. Zimmerman, K. Teeter and G. Gibson, 2000. Genetic variation for the positioning of wing veins in Drosophila melanogaster. Evol. Dev. 2: 16–24. - PubMed
-
- Bookstein, F. L., 1991. Morphometric Tools for Landmark Data: Geometry and Biology. Cambridge University Press, Cambridge, UK.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

