Syndapin I is the phosphorylation-regulated dynamin I partner in synaptic vesicle endocytosis
- PMID: 16648848
- PMCID: PMC2082060
- DOI: 10.1038/nn1695
Syndapin I is the phosphorylation-regulated dynamin I partner in synaptic vesicle endocytosis
Abstract
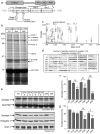
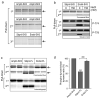
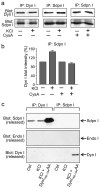
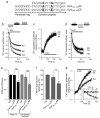
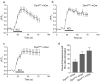
Dynamin I is dephosphorylated at Ser-774 and Ser-778 during synaptic vesicle endocytosis (SVE) in nerve terminals. Phosphorylation was proposed to regulate the assembly of an endocytic protein complex with amphiphysin or endophilin. Instead, we found it recruits syndapin I for SVE and does not control amphiphysin or endophilin binding in rat synaptosomes. After depolarization, syndapin showed a calcineurin-mediated interaction with dynamin. A peptide mimicking the phosphorylation sites disrupted the dynamin-syndapin complex, not the dynamin-endophilin complex, arrested SVE and produced glutamate release fatigue after repetitive stimulation. Pseudophosphorylation of Ser-774 or Ser-778 inhibited syndapin binding without affecting amphiphysin recruitment. Site mutagenesis to alanine arrested SVE in cultured neurons. The effects of the sites were additive for syndapin I binding and SVE. Thus syndapin I is a central component of the endocytic protein complex for SVE via stimulus-dependent recruitment to dynamin I and has a key role in synaptic transmission.
Figures

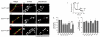
References
-
- Cousin MA, Robinson PJ. The dephosphins: Dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. - PubMed
-
- Tan TC, et al. Cdk5 is essential for synaptic vesicle endocytosis. Nat. Cell Biol. 2003;5:701–710. - PubMed
-
- Floyd SR, et al. Amphiphysin binds the cdk5 regulatory subunit p35 and is phosphorylated by cdk5 and cdc2. J. Biol. Chem. 2001;276:8104–8110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

