Biochemical markers of bone turnover: part I: biochemistry and variability
- PMID: 16648882
- PMCID: PMC1320175
Biochemical markers of bone turnover: part I: biochemistry and variability
Abstract
With the ageing population in most countries, disorders of bone and mineral metabolism are becoming increasingly relevant to every day clinical practice. Consequently, the interest in, and the need for effective measures to be used in the screening, diagnosis and follow-up of such pathologies has markedly grown. Together with clinical and imaging techniques, biochemical tests play an important role in the assessment and differential diagnosis of metabolic bone disease. In recent years, the isolation and characterisation of cellular and extracellular components of the skeletal matrix have resulted in the development of molecular markers that are considered to reflect either bone formation or bone resorption. These biochemical indices are non-invasive, comparatively inexpensive and, when applied and interpreted correctly, helpful tools in the diagnostic and therapeutic assessment of metabolic bone disease. Part I of this article provides an overview of the basic biochemistry of bone markers, and sources of non-specific variability. Part II (to be published in a subsequent issue of this journal) will review the current evidence regarding the clinical use of biochemical markers of bone remodelling in metabolic and metastatic bone disease.
Figures
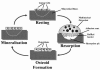












References
-
- Lian JB and Stein GS. The cells of bone. In: Dynamics of bone and cartilage metabolism. Seibel MJ, Robins SP, Bilezikian JP (eds), Academic Press, San Diego; 1999, 165–86.
-
- Rizzoli R and Bonjour JP. Physiology of calcium and phosphate homeostasis. In: Dynamics of bone and cartilage metabolism. Seibel MJ, Robins SP, Bilezikian JP (eds), Academic Press, San Diego; 1999, 247–60.
-
- Stinson RA, Hamilton BA. Human liver plasma membranes contain an enzyme activity that removes membrane anchor from alkaline phosphatase and converts it to a plasma-like form. Clin Biochem. 1994;27:49–55. - PubMed
-
- Harris H. The human alkaline phosphatases: what we know and what we don’t know. Clin Chim Acta. 1990;186:133–50. - PubMed
-
- Crofton PM. Biochemistry of alkaline phosphatase isoenzymes. Crit Rev Clin Lab Sci. 1982;16:161–94. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
