Evidence that membrane insertion of the cytosolic domain of Bcl-xL is governed by an electrostatic mechanism
- PMID: 16650855
- PMCID: PMC1785297
- DOI: 10.1016/j.jmb.2006.03.052
Evidence that membrane insertion of the cytosolic domain of Bcl-xL is governed by an electrostatic mechanism
Abstract
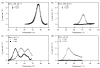
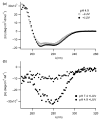
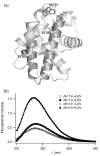
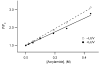
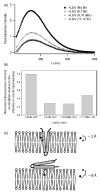
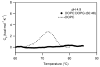
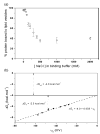
Signals from different cellular networks are integrated at the mitochondria in the regulation of apoptosis. This integration is controlled by the Bcl-2 proteins, many of which change localization from the cytosol to the mitochondrial outer membrane in this regulation. For Bcl-xL, this change in localization reflects the ability to undergo a conformational change from a solution to integral membrane conformation. To characterize this conformational change, structural and thermodynamic measurements were performed in the absence and presence of lipid vesicles with Bcl-xL. A pH-dependent model is proposed for the solution to membrane conformational change that consists of three stable conformations: a solution conformation, a conformation similar to the solution conformation but anchored to the membrane by its C-terminal transmembrane domain, and a membrane conformation that is fully associated with the membrane. This model predicts that the solution to membrane conformational change is independent of the C-terminal transmembrane domain, which is experimentally demonstrated. The conformational change is associated with changes in secondary and, especially, tertiary structure of the protein, as measured by far and near-UV circular dichroism spectroscopy, respectively. Membrane insertion was distinguished from peripheral association with the membrane by quenching of intrinsic tryptophan fluorescence by acrylamide and brominated lipids. For the cytosolic domain, the free energy of insertion (DeltaG degrees x) into lipid vesicles was determined to be -6.5 kcal mol(-1) at pH 4.9 by vesicle binding experiments. To test whether electrostatic interactions were significant to this process, the salt dependence of this conformational change was measured and analyzed in terms of Gouy-Chapman theory to estimate an electrostatic contribution of DeltaG degrees el approximately -2.5 kcal mol(-1) and a non-electrostatic contribution of DeltaG degrees nel approximately -4.0 kcal mol(-1) to the free energy of insertion, DeltaG degrees x. Calcium, which blocks ion channel activity of Bcl-xL, did not affect the solution to membrane conformational change more than predicted by these electrostatic considerations. The lipid cardiolipin, that is enriched at mitochondrial contact sites and reported to be important for the localization of Bcl-2 proteins, did not affect the solution to membrane conformational change of the cytosolic domain, suggesting that this lipid is not involved in the localization of Bcl-xL in vivo. Collectively, these data suggest the solution to membrane conformational change is controlled by an electrostatic mechanism. Given the distinct biological activities of these conformations, the possibility that this conformational change might be a regulatory checkpoint for apoptosis is discussed.
Figures

References
-
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. - PubMed
-
- Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 2003;15:691–699. - PubMed
-
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. - PubMed
-
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. - PubMed
-
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

