Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes
- PMID: 16651469
- PMCID: PMC1877795
- DOI: 10.1161/CIRCULATIONAHA.105.599241
Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes
Abstract
Background: Cyclic guanosine monophosphate (cGMP) is the common second messenger for the cardiovascular effects of nitric oxide (NO) and natriuretic peptides, such as atrial or brain natriuretic peptide, which activate the soluble and particulate forms of guanylyl cyclase, respectively. However, natriuretic peptides and NO donors exert different effects on cardiac and vascular smooth muscle function. We therefore tested whether these differences are due to an intracellular compartmentation of cGMP and evaluated the role of phosphodiesterase (PDE) subtypes in this process.
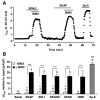
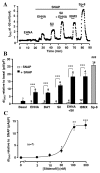
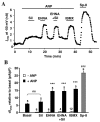
Methods and results: Subsarcolemmal cGMP signals were monitored in adult rat cardiomyocytes by expression of the rat olfactory cyclic nucleotide-gated (CNG) channel alpha-subunit and recording of the associated cGMP-gated current (ICNG). Atrial natriuretic peptide (10 nmol/L) or brain natriuretic peptide (10 nmol/L) induced a clear activation of ICNG, whereas NO donors (S-nitroso-N-acetyl-penicillamine, diethylamine NONOate, 3-morpholinosydnonimine, and spermine NO, all at 100 micromol/L) had little effect. The ICNG current was strongly potentiated by nonselective PDE inhibition with isobutyl methylxanthine (100 micromol/L) and by the PDE2 inhibitors erythro-9-(2-hydroxy-3-nonyl)adenine (10 micromol/L) and Bay 60-7550 (50 nmol/L). Surprisingly, sildenafil, a PDE5 inhibitor, produced a dose-dependent increase of I(CNG) activated by NO donors but had no effect (at 100 nmol/L) on the current elicited by atrial natriuretic peptide.
Conclusions: These results indicate that in rat cardiomyocytes (1) the particulate cGMP pool is readily accessible at the plasma membrane, whereas the soluble pool is not; and (2) PDE5 controls the soluble but not the particulate pool, whereas the latter is under the exclusive control of PDE2. Differential spatiotemporal distributions of cGMP may therefore contribute to the specific effects of natriuretic peptides and NO donors on cardiac function.
Figures


References
-
- Shah AM, MacCarthy PA. Paracrine and autocrine effects of nitric oxide on myocardial function. Pharmacol Ther. 2000;86:49–86. - PubMed
-
- Semigran MJ. Type 5 phosphodiesterase inhibition: the focus shifts to the heart. Circulation. 2005;112:2589–2591. - PubMed
-
- Rosenkranz AC, Woods RL, Dusting GJ, Ritchie RH. Antihypertrophic actions of the natriuretic peptides in adult rat cardiomyocytes: importance of cyclic GMP. Cardiovasc Res. 2003;57:515–522. - PubMed
-
- Kempf T, Wollert KC. Nitric oxide and the enigma of cardiac hypertrophy. BioEssays. 2004;26:608–615. - PubMed
-
- Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

