Ongoing coxsackievirus myocarditis is associated with increased formation and activity of myocardial immunoproteasomes
- PMID: 16651621
- PMCID: PMC1606581
- DOI: 10.2353/ajpath.2006.050865
Ongoing coxsackievirus myocarditis is associated with increased formation and activity of myocardial immunoproteasomes
Abstract
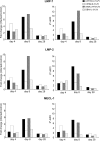
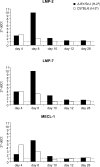
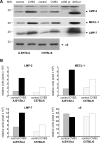
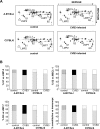
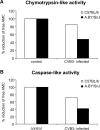
A growing body of evidence indicates that viral infections of the heart contribute to ongoing myocarditis and dilated cardiomyopathy. Murine models of coxsackievirus B3 (CVB3)-induced myocarditis mimic the human disease and allow identification of susceptibility factors that modulate the course of viral myocarditis. Susceptible mouse strains develop chronic myocarditis on the basis of restricted viral replication, whereas resistant strains recover after successful virus elimination. In comparative whole-genome microarray analyses of infected hearts, several genes involved in the processing and presentation of viral epitopes were found to be uniformly up-regulated in acutely CVB3-infected susceptible mice compared with resistant animals. In particular, expression of the catalytic subunits LMP2, LMP7, and MECL-1, immunoproteasome proteins important in the generation of major histocom-patibility complex (MHC) class I-restricted peptides, was clearly enhanced in the susceptible host. Increased expression resulted in enhanced formation of immunoproteasomes and altered proteolytic activities of proteasomes in the heart. This was accompanied by a concerted up-regulation of the antigen-presenting machinery in susceptible mice. Thus, we propose that increased formation of immunoproteasomes in susceptible mice affects the generation of antigenic peptides and the subsequent T-cell-mediated immune responses.
Figures

Similar articles
-
Differential interferon responses enhance viral epitope generation by myocardial immunoproteasomes in murine enterovirus myocarditis.Am J Pathol. 2009 Aug;175(2):510-8. doi: 10.2353/ajpath.2009.090033. Epub 2009 Jul 9. Am J Pathol. 2009. PMID: 19590042 Free PMC article.
-
PA28 modulates antigen processing and viral replication during coxsackievirus B3 infection.PLoS One. 2017 Mar 9;12(3):e0173259. doi: 10.1371/journal.pone.0173259. eCollection 2017. PLoS One. 2017. PMID: 28278207 Free PMC article.
-
The outcome of coxsackievirus B3-(CVB3-) induced myocarditis is influenced by the cellular immune status.Herz. 2000 May;25(3):245-8. doi: 10.1007/s000590050014. Herz. 2000. PMID: 10904846
-
Intricacies of cardiac damage in coxsackievirus B3 infection: implications for therapy.Int J Cardiol. 2014 Dec 15;177(2):330-339. doi: 10.1016/j.ijcard.2014.09.136. Epub 2014 Oct 18. Int J Cardiol. 2014. PMID: 25449464 Free PMC article. Review.
-
What lessons can be learned from animal model studies in viral heart disease?Scand J Infect Dis Suppl. 1993;88:49-65. Scand J Infect Dis Suppl. 1993. PMID: 8390719 Review.
Cited by
-
Interferon-dependent immunoproteasome activity during mouse adenovirus type 1 infection.Virology. 2016 Nov;498:57-68. doi: 10.1016/j.virol.2016.08.009. Epub 2016 Aug 22. Virology. 2016. PMID: 27560373 Free PMC article.
-
Silencing the CSF-1 Axis Using Nanoparticle Encapsulated siRNA Mitigates Viral and Autoimmune Myocarditis.Front Immunol. 2018 Oct 8;9:2303. doi: 10.3389/fimmu.2018.02303. eCollection 2018. Front Immunol. 2018. PMID: 30349538 Free PMC article.
-
Cytokine-induced oxidative stress in cardiac inflammation and heart failure-how the ubiquitin proteasome system targets this vicious cycle.Front Physiol. 2013 Mar 6;4:42. doi: 10.3389/fphys.2013.00042. eCollection 2013. Front Physiol. 2013. PMID: 23508734 Free PMC article.
-
Differential interferon responses enhance viral epitope generation by myocardial immunoproteasomes in murine enterovirus myocarditis.Am J Pathol. 2009 Aug;175(2):510-8. doi: 10.2353/ajpath.2009.090033. Epub 2009 Jul 9. Am J Pathol. 2009. PMID: 19590042 Free PMC article.
-
Protein degradation systems in viral myocarditis leading to dilated cardiomyopathy.Cardiovasc Res. 2010 Jan 15;85(2):347-56. doi: 10.1093/cvr/cvp225. Epub 2009 Jul 3. Cardiovasc Res. 2010. PMID: 19578074 Free PMC article. Review.
References
-
- Mason JW. Myocarditis and dilated cardiomyopathy: an inflammatory link. Cardiovasc Res. 2003;60:5–10. - PubMed
-
- Kuehl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, Poller W, Kandolf R, Schultheiss HP. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation. 2005;111:887–893. - PubMed
-
- Huber SA, Gauntt CJ, Sakkinen P. Enteroviruses and myocarditis: viral pathogenesis through replication, cytokine induction, and immunopathogenicity. Adv Virus Res. 1998;51:35–80. - PubMed
-
- Klingel K, Sauter M, Bock CT, Szalay G, Schnorr JJ, Kandolf R. Molecular pathology of inflammatory cardiomyopathy. Med Microbiol Immunol. 2004;193:101–107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

