Beta-amyloid mediated nitration of manganese superoxide dismutase: implication for oxidative stress in a APPNLH/NLH X PS-1P264L/P264L double knock-in mouse model of Alzheimer's disease
- PMID: 16651627
- PMCID: PMC1606606
- DOI: 10.2353/ajpath.2006.051223
Beta-amyloid mediated nitration of manganese superoxide dismutase: implication for oxidative stress in a APPNLH/NLH X PS-1P264L/P264L double knock-in mouse model of Alzheimer's disease
Abstract
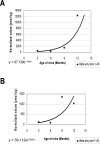
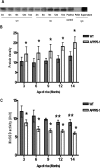
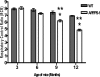
Alzheimer's disease is a multifactorial, progressive, age-related neurodegenerative disease. In familial Alzheimer's disease, Abeta is excessively produced and deposited because of mutations in the amyloid precursor protein, presenilin-1, and presenilin-2 genes. Here, we generated a double homozygous knock-in mouse model that incorporates the Swedish familial Alzheimer's disease mutations and converts mouse Abeta to the human sequence in amyloid precursor protein and had the P264L familial Alzheimer's disease mutation in presenilin-1. We observed Abeta deposition in double knock-in mice beginning at 6 months as well as an increase in the levels of insoluble Abeta1-40/1-42. Brain homogenates from 3-, 6-, 9-, 12-, and 14-month-old mice showed that protein levels of manganese superoxide dismutase (MnSOD) were unchanged in the double knock-in mice compared to controls. Genotype-associated increases in nitrotyrosine levels were observed. Protein immunoprecipitation revealed MnSOD as a target of this nitration. Although the levels of MnSOD protein did not change, MnSOD activity and mitochondrial respiration decreased in knock-in mice, suggesting compromised mitochondrial function. The compromised activity of MnSOD, a primary antioxidant enzyme protecting mitochondria, may explain mitochondrial dysfunction and provide the missing link between Abeta-induced oxidative stress and Alzheimer's disease.
Figures


References
-
- Evans DA, Funkenstein HH, Albert MS, Scherr PA, Cook NR, Chown MJ, Hebert LE, Hennekens CH, Taylor JO. Prevalence of Alzheimer’s disease in a community population of older people. JAMA. 1989;262:2551–2556. - PubMed
-
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of Aβ 42(43) and Aβ 40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ 42(43). Neuron. 1994;13:45–53. - PubMed
-
- Selkoe DJ. Clearing the brain’s amyloid cobwebs. Neuron. 2001;25:177–180. - PubMed
-
- Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32:1050–1060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

