SOX2 is a dose-dependent regulator of retinal neural progenitor competence
- PMID: 16651659
- PMCID: PMC1472477
- DOI: 10.1101/gad.1407906
SOX2 is a dose-dependent regulator of retinal neural progenitor competence
Abstract
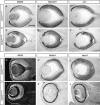
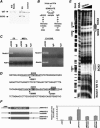
Approximately 10% of humans with anophthalmia (absent eye) or severe microphthalmia (small eye) show haploid insufficiency due to mutations in SOX2, a SOXB1-HMG box transcription factor. However, at present, the molecular or cellular mechanisms responsible for these conditions are poorly understood. Here, we directly assessed the requirement for SOX2 during eye development by generating a gene-dosage allelic series of Sox2 mutations in the mouse. The Sox2 mutant mice display a range of eye phenotypes consistent with human syndromes and the severity of these phenotypes directly relates to the levels of SOX2 expression found in progenitor cells of the neural retina. Retinal progenitor cells with conditionally ablated Sox2 lose competence to both proliferate and terminally differentiate. In contrast, in Sox2 hypomorphic/null mice, a reduction of SOX2 expression to <40% of normal causes variable microphthalmia as a result of aberrant neural progenitor differentiation. Furthermore, we provide genetic and molecular evidence that SOX2 activity, in a concentration-dependent manner, plays a key role in the regulation of the NOTCH1 signaling pathway in retinal progenitor cells. Collectively, these results show that precise regulation of SOX2 dosage is critical for temporal and spatial regulation of retinal progenitor cell differentiation and provide a cellular and molecular model for understanding how hypomorphic levels of SOX2 cause retinal defects in humans.
Figures






Similar articles
-
The master transcription factor SOX2, mutated in anophthalmia/microphthalmia, is post-transcriptionally regulated by the conserved RNA-binding protein RBM24 in vertebrate eye development.Hum Mol Genet. 2020 Mar 13;29(4):591-604. doi: 10.1093/hmg/ddz278. Hum Mol Genet. 2020. PMID: 31814023 Free PMC article.
-
SOX2 anophthalmia syndrome: 12 new cases demonstrating broader phenotype and high frequency of large gene deletions.Br J Ophthalmol. 2007 Nov;91(11):1471-6. doi: 10.1136/bjo.2007.117929. Epub 2007 May 23. Br J Ophthalmol. 2007. PMID: 17522144 Free PMC article.
-
Establishment of the neurogenic boundary of the mouse retina requires cooperation of SOX2 and WNT signaling.Neural Dev. 2014 Dec 9;9:27. doi: 10.1186/1749-8104-9-27. Neural Dev. 2014. PMID: 25488119 Free PMC article.
-
[SOX2 defect and anophthalmia and microphthalmia].Zhonghua Yan Ke Za Zhi. 2012 Nov;48(11):1049-52. Zhonghua Yan Ke Za Zhi. 2012. PMID: 23302280 Review. Chinese.
-
SOX2 functions in adult neural stem cells.Trends Neurosci. 2005 May;28(5):219-21. doi: 10.1016/j.tins.2005.03.003. Trends Neurosci. 2005. PMID: 15866195 Review.
Cited by
-
The initial establishment and epithelial morphogenesis of the esophagus: a new model of tracheal-esophageal separation and transition of simple columnar into stratified squamous epithelium in the developing esophagus.Wiley Interdiscip Rev Dev Biol. 2015 Jul-Aug;4(4):419-30. doi: 10.1002/wdev.179. Epub 2015 Feb 27. Wiley Interdiscip Rev Dev Biol. 2015. PMID: 25727889 Free PMC article.
-
Overlapping expression patterns and redundant roles for AP-2 transcription factors in the developing mammalian retina.Dev Dyn. 2012 Apr;241(4):814-29. doi: 10.1002/dvdy.23762. Dev Dyn. 2012. PMID: 22411557 Free PMC article.
-
Non-Cell Autonomous Roles for CASK in Optic Nerve Hypoplasia.Invest Ophthalmol Vis Sci. 2019 Aug 1;60(10):3584-3594. doi: 10.1167/iovs.19-27197. Invest Ophthalmol Vis Sci. 2019. PMID: 31425583 Free PMC article.
-
RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus.J Neurosci. 2010 Oct 13;30(41):13794-807. doi: 10.1523/JNEUROSCI.1567-10.2010. J Neurosci. 2010. PMID: 20943920 Free PMC article.
-
Six3 and Six6 Are Jointly Required for the Maintenance of Multipotent Retinal Progenitors through Both Positive and Negative Regulation.Cell Rep. 2018 Nov 27;25(9):2510-2523.e4. doi: 10.1016/j.celrep.2018.10.106. Cell Rep. 2018. PMID: 30485816 Free PMC article.
References
-
- Ahmad I., Dooley C.M., Polk D.L. Delta-1 is a regulator of neurogenesis in the vertebrate retina. Dev. Biol. 1997;185:92–103. - PubMed
-
- Austin C.P., Feldman D.E., Ida J.A., Jr., Cepko C.L. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development. 1995;121:3637–3650. - PubMed
-
- Bylund M., Andersson E., Novitch B.G., Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat. Neurosci. 2003;6:1162–1168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
