A drug-sensitive genetic network masks fungi from the immune system
- PMID: 16652171
- PMCID: PMC1447670
- DOI: 10.1371/journal.ppat.0020035
A drug-sensitive genetic network masks fungi from the immune system
Abstract
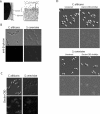
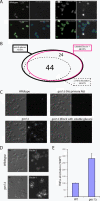
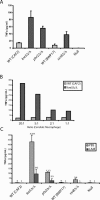
Fungal pathogens can be recognized by the immune system via their beta-glucan, a potent proinflammatory molecule that is present at high levels but is predominantly buried beneath a mannoprotein coat and invisible to the host. To investigate the nature and significance of "masking" this molecule, we characterized the mechanism of masking and consequences of unmasking for immune recognition. We found that the underlying beta-glucan in the cell wall of Candida albicans is unmasked by subinhibitory doses of the antifungal drug caspofungin, causing the exposed fungi to elicit a stronger immune response. Using a library of bakers' yeast (Saccharomyces cerevisiae) mutants, we uncovered a conserved genetic network that is required for concealing beta-glucan from the immune system and limiting the host response. Perturbation of parts of this network in the pathogen C. albicans caused unmasking of its beta-glucan, leading to increased beta-glucan receptor-dependent elicitation of key proinflammatory cytokines from primary mouse macrophages. By creating an anti-inflammatory barrier to mask beta-glucan, opportunistic fungi may promote commensal colonization and have an increased propensity for causing disease. Targeting the widely conserved gene network required for creating and maintaining this barrier may lead to novel broad-spectrum antimycotics.
Conflict of interest statement
Figures



References
-
- Underhill DM, Ozinsky A. Phagocytosis of microbes: Complexity in action. Annu Rev Immunol. 2002;20:825–852. - PubMed
-
- Hajjeh RA, Sofair AN, Harrison LH, Lyon GM, Arthington-Skaggs BA, et al. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J Clin Microbiol. 2004;42:1519–1527. - PMC - PubMed
-
- Poulain D, Jouault T. Candida albicans cell wall glycans, host receptors and responses: Elements for a decisive crosstalk. Curr Opin Microbiol. 2004;7:342–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

