Extrinsic interactions dominate helical propensity in coupled binding and folding of the lactose repressor protein hinge helix
- PMID: 16669632
- PMCID: PMC2701349
- DOI: 10.1021/bi052619p
Extrinsic interactions dominate helical propensity in coupled binding and folding of the lactose repressor protein hinge helix
Abstract
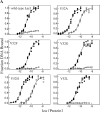
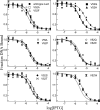
A significant number of eukaryotic regulatory proteins are predicted to have disordered regions. Many of these proteins bind DNA, which may serve as a template for protein folding. Similar behavior is seen in the prokaryotic LacI/GalR family of proteins that couple hinge-helix folding with DNA binding. These hinge regions form short alpha-helices when bound to DNA but appear to be disordered in other states. An intriguing question is whether and to what degree intrinsic helix propensity contributes to the function of these proteins. In addition to its interaction with operator DNA, the LacI hinge helix interacts with the hinge helix of the homodimer partner as well as to the surface of the inducer-binding domain. To explore the hierarchy of these interactions, we made a series of substitutions in the LacI hinge helix at position 52, the only site in the helix that does not interact with DNA and/or the inducer-binding domain. The substitutions at V52 have significant effects on operator binding affinity and specificity, and several substitutions also impair functional communication with the inducer-binding domain. Results suggest that helical propensity of amino acids in the hinge region alone does not dominate function; helix-helix packing interactions appear to also contribute. Further, the data demonstrate that variation in operator sequence can overcome side chain effects on hinge-helix folding and/or hinge-hinge interactions. Thus, this system provides a direct example whereby an extrinsic interaction (DNA binding) guides internal events that influence folding and functionality.
Figures
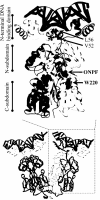
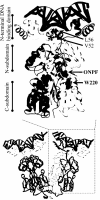







References
-
- Matthews KS, Nichols JC. Lactose repressor protein: Functional properties and structure. Prog. Nucleic Acid Res. Mol. Biol. 1998;58:127–164. - PubMed
-
- Straney DC, Crothers DM. Effect of drug-DNA interactions upon transcription initiation at the lac promoter. Biochemistry. 1987;26:1987–1995. - PubMed
-
- Lin S-Y, Riggs AD. A comparison of lac repressor binding to operator and to nonoperator DNA. Biochem. Biophys. Res. Commun. 1975;62:704–710. - PubMed
-
- Jobe A, Bourgeois S. lac repressor-operator interaction. VI. The natural inducer of the lac operon. J. Mol. Biol. 1972;69:397–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

