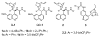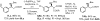The Mannich reaction of malonates with simple imines catalyzed by bifunctional cinchona alkaloids: enantioselective synthesis of beta-amino acids
- PMID: 16669669
- PMCID: PMC3172002
- DOI: 10.1021/ja060716f
The Mannich reaction of malonates with simple imines catalyzed by bifunctional cinchona alkaloids: enantioselective synthesis of beta-amino acids
Abstract
We describe the first efficient, direct asymmetric Mannich reactions with malonates and N-Boc aryl and alkyl imines by cooperative hydrogen-bonding catalysis with a cinchona alkaloid bearing a thiourea functionality. We have also extended the scope of this reaction to beta-ketoesters. The synthetic value of this new reaction is demonstrated in the establishment of a convergent enantioselective route toward the biologically important beta-amino acids under mild and air- and moisture-tolerant conditions.
Figures
References
-
-
For reviews, see: Córdova A. Acc Chem Res. 2004;37:102.Kobayashi S, Ueno M. In: Comprehensive Asymmetric Catalysis Supplement I. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Chapter 29.5 Springer; Berlin: 2003. Kobayashi S, Ishitani H. Chem Rev. 1999;99:1069.and reference therein.
-
-
- Kobayashi S, Ueno M, Saito S, Mizuki Y, Ishitani H, Yamashita Y. Proc Natl Acad Sci USA. 2004;101:5476. - PMC - PubMed
- Akiyama T, Itoh J, Yokota K, Fuchibe K. Angew Chem Int Ed. 2004;43:1566. - PubMed
- Josephsohn NS, Snapper ML, Hoveyda AH. J Am Chem Soc. 2004;126:3734. - PubMed
- Kobayashi S, Matsubara R, Nakamura Y, Kitagawa H, Sugiura M. J Am Chem Soc. 2003;125:2507. - PubMed
- Wenzel AG, Lalonde MP, Jacobsen EN. Synlett. 2003:1919.
- Wenzel AG, Jacobsen EN. J Am Chem Soc. 2002;124:12964. - PubMed
- Kobayashi S, Hamada T, Manabe K. J Am Chem Soc. 2002;124:5640. - PubMed
-
- Lou S, Taoka BM, Ting A, Schaus SE. J Am Chem Soc. 2005;127:11256. - PubMed
- Hamashima Y, Sasamoto N, Hotta D, Somei H, Umebayashi N, Sodeoka M. Angew Chem Int Ed. 2005;44:1525. - PubMed
- Poulsen TB, Alemparte C, Saaby S, Bella M, Jørgensen KA. Angew Chem Int Ed. 2005;44:2. - PubMed
- Uraguchi D, Terada M. J Am Chem Soc. 2004;126:5356. - PubMed
-
- Trost BM, Jaratjaroonphong J, Reutrakul V. J Am Chem Soc. 2006;128:2778. - PMC - PubMed
- Mitsumori S, Zhang H, Cheong P, Houk KN, Tanaka F, Barbas CF., III J Am Chem Soc. 2006;128:1040. - PMC - PubMed
- Kano T, Yamaguchi Y, Tokuda O, Maruoka K. J Am Chem Soc. 2005;127:16408. - PubMed
- Harada S, Handa S, Matsunaga S, Shibasaki M. Angew Chem Int Ed. 2005;44:4365. - PubMed
- Okada A, Shibuguchi T, Ohshima T, Masu H, Yamaguchi K, Shibasaki M. Angew Chem Int Ed. 2005;44:4564. - PubMed
- Notz W, Watanabe SI, Chowdari NS, Zhong G, Betancort JM, Tanaka F, Barbas CF., III Adv Synth Catal. 2004;346:1131.
- Zhuang W, Saaby S, Jørgensen KA. Angew Chem Int Ed. 2004;43:4476. - PubMed
- Córdova A. Chem Eur J. 2004;10:1987. - PubMed
- Notz W, Tanaka F, Barbas CF., III Acc Chem Res. 2004;37:5801. - PubMed
- Hayashi Y, Tsuboi W, Ashimine I, Urushima T, Shoji M, Sakai K. Angew Chem Int Ed. 2003;42:3805. - PubMed
- List B, Pojarliev P, Biller WT, Martin HJ. J Am Chem Soc. 2002;124:827. - PubMed
- List B. J Am Chem Soc. 2000;122:9336.
-
-
Only one Mannich reaction with malonates and an activated N-tosyl-α-imino ester in 39–87% ee has been reported: Marigo M, Kjærsgaard A, Juhl K, Gathergood N, Jørgensen KA. Chem Eur J. 2003;9:2359.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources