New class of nucleophiles for palladium-catalyzed asymmetric allylic alkylation. Total synthesis of agelastatin A
- PMID: 16669672
- PMCID: PMC2585983
- DOI: 10.1021/ja061105q
New class of nucleophiles for palladium-catalyzed asymmetric allylic alkylation. Total synthesis of agelastatin A
Abstract
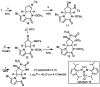
New classes of nucleophiles, pyrroles, and N-methoxyamides were developed for Pd-catalyzed AAA reactions. By varying the functional groups at the 2-position of pyrroles, either regioisomer of the piperazinone is available. Using one regioisomer, the total synthesis of (+)-agelastatin A in 10 total steps is accomplished. For this synthesis, a new copper-catalyzed aziridination and an indium-catalyzed oxidative ring opening of a N-tosylaziridine were developed. The feasibility of accessing (-)-agelastatin A from the same enantiomer of the chiral catalyst from the other regioisomeric piperazinone is indicated.
Figures
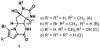
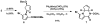
References
-
-
Cordell GA, editor. The Alkaloids. Chemistry and Biology. Academic Press; San Diego: 2005. p. 62. and earlier volumes in the series. Hesse M. Alkaloids: Nature’s Curse or Blessing. Wiley-VCH; New York: 2002.
-
-
-
For a recent review: Trost BM, Crawley ML. Chem Rev. 2003;103:2921. Also see Trost BM. Chem Pharm Bull. 2004;50:1.
-
-
-
Gribble GW. J Nat Prod. 1992;55:1353.Also see Faulkner DJ. Nat Prod Rep. 2002;19:1.Berlinck RGS, Kosuga MH. Nat Prod Rep. 2005;22:516. and references therein.
-
-
-
For the isolation and biological activities of (−)-agelastatin A: D’Ambrosio M, Guerriero A, Debitus C, Ribes O, Pusset J, Leroy S, Pietra F. J Chem Soc, Chem Comm. 1993;1305D’Ambrosio M, Guerriero A, Chiasera G, Pietra F. Helv Chim Acta. 1994;1895;77D’Ambrosio M, Guerriero A, Ripamonti M, Debitus C, Waikedre J, Pietra F. Helv Chim Acta. 1996;79:727.Maijer L, Thunnissen AM, White AW, Garnier M, Nikolic M, Tsai LH, Walter J, Cleverley KE, Salinas PC, Wu YZ, Biernat J, Mandelkov EM, Kim SH, Pettit GR. Chem Bio. 2000;2:51.
-
-
-
Reagents that have been tried: AlMe3, ClMgiPr, KCN, MgCl2, Zr(OBut)4, an N-heterocyclic carbene, Sn[N(TMS)2]2, etc.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

