Climate, deer, rodents, and acorns as determinants of variation in lyme-disease risk
- PMID: 16669698
- PMCID: PMC1457019
- DOI: 10.1371/journal.pbio.0040145
Climate, deer, rodents, and acorns as determinants of variation in lyme-disease risk
Abstract
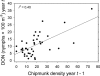
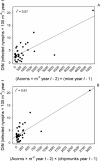
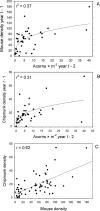
Risk of human exposure to vector-borne zoonotic pathogens is a function of the abundance and infection prevalence of vectors. We assessed the determinants of Lyme-disease risk (density and Borrelia burgdorferi-infection prevalence of nymphal Ixodes scapularis ticks) over 13 y on several field plots within eastern deciduous forests in the epicenter of US Lyme disease (Dutchess County, New York). We used a model comparison approach to simultaneously test the importance of ambient growing-season temperature, precipitation, two indices of deer (Odocoileus virginianus) abundance, and densities of white-footed mice (Peromyscus leucopus), eastern chipmunks (Tamias striatus), and acorns (Quercus spp.), in both simple and multiple regression models, in predicting entomological risk. Indices of deer abundance had no predictive power, and precipitation in the current year and temperature in the prior year had only weak effects on entomological risk. The strongest predictors of a current year's risk were the prior year's abundance of mice and chipmunks and abundance of acorns 2 y previously. In no case did inclusion of deer or climate variables improve the predictive power of models based on rodents, acorns, or both. We conclude that interannual variation in entomological risk of exposure to Lyme disease is correlated positively with prior abundance of key hosts for the immature stages of the tick vector and with critical food resources for those hosts.
Figures



Comment in
-
A new view on Lyme disease: rodents hold the key to annual risk.PLoS Biol. 2006 Jun;4(6):e182. doi: 10.1371/journal.pbio.0040182. Epub 2006 May 9. PLoS Biol. 2006. PMID: 20076583 Free PMC article. No abstract available.
References
-
- Antonovics J, Iwasa Y, Hassell M. A generalized model of parasitoid, venereal, and vector-based transmission processes. Am Nat. 1995;145:661–675.
-
- Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson A, et al. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. - PubMed
-
- Hubalek Z. North Atlantic weather oscillation and human infectious diseases in the Czech Republic, 1951–2003. Eur J Epidemiol. 2005;20:263–270. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

