Chronic Obstructive Pulmonary Disease, inflammation and co-morbidity--a common inflammatory phenotype?
- PMID: 16669999
- PMCID: PMC1479815
- DOI: 10.1186/1465-9921-7-70
Chronic Obstructive Pulmonary Disease, inflammation and co-morbidity--a common inflammatory phenotype?
Abstract
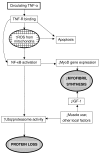
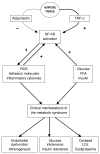
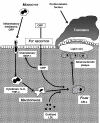
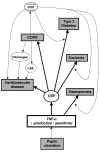
Chronic Obstructive Pulmonary Disease (COPD) is and will remain a major cause of morbidity and mortality worldwide. The severity of airflow obstruction is known to relate to overall health status and mortality. However, even allowing for common aetiological factors, a link has been identified between COPD and other systemic diseases such as cardiovascular disease, diabetes and osteoporosis. COPD is known to be an inflammatory condition and neutrophil elastase has long been considered a significant mediator of the disease. Pro-inflammatory cytokines, in particular TNF-alpha (Tumour Necrosis Factor alpha), may be the driving force behind the disease process. However, the roles of inflammation and these pro-inflammatory cytokines may extend beyond the lungs and play a part in the systemic effects of the disease and associated co-morbidities. This article describes the mechanisms involved and proposes a common inflammatory TNF-alpha phenotype that may, in part, account for the associations.
Figures
References
-
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. - PubMed
-
- Ferrer M, Alonso J, Morera J, Marrades RM, Khalaf A, Aguar MC, Plaza V, Prieto L, Anto JM. Chronic obstructive pulmonary disease stage and health-related quality of life. The Quality of Life of Chronic Obstructive Pulmonary Disease Study Group. Ann Intern Med. 1997;127:1072–1079. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

