Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha)-deficient mice
- PMID: 16670093
- PMCID: PMC3047410
- DOI: 10.1074/jbc.M600050200
Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha)-deficient mice
Abstract
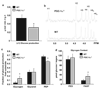
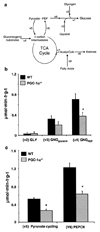
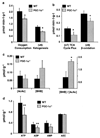
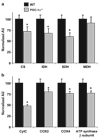
The peroxisome proliferator-activated receptor gamma (PPARgamma) coactivator 1alpha (PGC-1alpha) is a highly inducible transcriptional coactivator implicated in the coordinate regulation of genes encoding enzymes involved in hepatic fatty acid oxidation, oxidative phosphorylation, and gluconeogenesis. The present study sought to assess the effects of chronic PGC-1alpha deficiency on metabolic flux through the hepatic gluconeogenic, fatty acid oxidation, and tricarboxylic acid cycle pathways. To this end, hepatic metabolism was assessed in wild-type (WT) and PGC-1alpha(-/-) mice using isotopomer-based NMR with complementary gene expression analyses. Hepatic glucose production was diminished in PGC-1alpha(-/-) livers coincident with reduced gluconeogenic flux from phosphoenolpyruvate. Surprisingly, the expression of PGC-1alpha target genes involved in gluconeogenesis was unaltered in PGC-1alpha(-/-) compared with WT mice under fed and fasted conditions. Flux through tricarboxylic acid cycle and mitochondrial fatty acid beta-oxidation pathways was also diminished in PGC-1alpha(-/-) livers. The expression of multiple genes encoding tricarboxylic acid cycle and oxidative phosphorylation enzymes was significantly depressed in PGC-1alpha(-/-) mice and was activated by PGC-1alpha overexpression in the livers of WT mice. Collectively, these findings suggest that chronic whole-animal PGC-1alpha deficiency results in defects in hepatic glucose production that are secondary to diminished fatty acid beta-oxidation and tricarboxylic acid cycle flux rather than abnormalities in gluconeogenic enzyme gene expression per se.
Figures



References
-
- Lin J, Handschin C, Spiegelman BM. Cell Metab. 2005;1:361–370. - PubMed
-
- Kelly DP, Scarpulla RC. Genes Dev. 2004;18:357–368. - PubMed
-
- Spiegelman BM, Heinrich R. Cell. 2004;119:157–167. - PubMed
-
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. Cell. 1998;92:829–839. - PubMed
-
- Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. Nature. 2001;413:179–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL058427/HL/NHLBI NIH HHS/United States
- K01 DK062903/DK/NIDDK NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- P30 DK56341/DK/NIDDK NIH HHS/United States
- R01 DK045416/DK/NIDDK NIH HHS/United States
- R01 HL58427/HL/NHLBI NIH HHS/United States
- RR02584/RR/NCRR NIH HHS/United States
- P01 HL057278/HL/NHLBI NIH HHS/United States
- R01 DK45416/DK/NIDDK NIH HHS/United States
- P01 HL57278/HL/NHLBI NIH HHS/United States
- U24-DK59632/DK/NIDDK NIH HHS/United States
- R01 HL101189/HL/NHLBI NIH HHS/United States
- P41 RR002584/RR/NCRR NIH HHS/United States
- U24 DK059632/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

