Growth hormone promotes skeletal muscle cell fusion independent of insulin-like growth factor 1 up-regulation
- PMID: 16670201
- PMCID: PMC1456062
- DOI: 10.1073/pnas.0510033103
Growth hormone promotes skeletal muscle cell fusion independent of insulin-like growth factor 1 up-regulation
Abstract
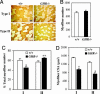
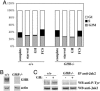
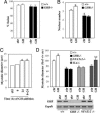
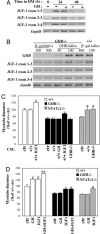
Growth hormone (GH) participates in the postnatal regulation of skeletal muscle growth, although the mechanism of action is unclear. Here we show that the mass of skeletal muscles lacking GH receptors is reduced because of a decrease in myofiber size with normal myofiber number. GH signaling controls the size of the differentiated myotubes in a cell-autonomous manner while having no effect on size, proliferation, and differentiation of the myoblast precursor cells. The GH hypertrophic action leads to an increased myonuclear number, indicating that GH facilitates fusion of myoblasts with nascent myotubes. NFATc2, a transcription factor regulating this phase of fusion, is required for GH action because GH is unable to induce hypertrophy of NFATc2-/- myotubes. Finally, we provide three lines of evidence suggesting that GH facilitates cell fusion independent of insulin-like growth factor 1 (IGF-1) up-regulation. First, GH does not regulate IGF-1 expression in myotubes; second, GH action is not mediated by a secreted factor in conditioned medium; third, GH and IGF-1 hypertrophic effects are additive and rely on different signaling pathways. Taken together, these data unravel a specific function of GH in the control of cell fusion, an essential process for muscle growth.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Florini J. R., Ewton D. Z., Coolican S. A. Endocr. Rev. 1996;17:481–517. - PubMed
-
- Hoffman A. R., Kuntze J. E., Baptista J., Baum H. B., Baumann G. P., Biller B. M., Clark R. V., Cook D., Inzucchi S. E., Kleinberg D., et al. J. Clin. Endocrinol. Metab. 2004;89:2048–2056. - PubMed
-
- Le Roith D., Bondy C., Yakar S., Liu J. L., Butler A. Endocr. Rev. 2001;22:53–74. - PubMed
-
- Shavlakadze T., Winn N., Rosenthal N., Grounds M. D. Growth Horm. IGF Res. 2005;15:4–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

