T-cell antigen-receptor stoichiometry: pre-clustering for sensitivity
- PMID: 16670682
- PMCID: PMC1479560
- DOI: 10.1038/sj.embor.7400682
T-cell antigen-receptor stoichiometry: pre-clustering for sensitivity
Abstract
The T-cell antigen receptor (TCR x CD3) is a multi-subunit complex that is responsible for triggering an adaptive immune response. It shows high specificity and sensitivity, while having a low affinity for the ligand. Furthermore, T cells respond to antigen over a wide concentration range. The stoichiometry and architecture of TCR x CD3 in the membrane have been under intense scrutiny because they might be the key to explaining its paradoxical properties. This review highlights new evidence that TCR x CD3 is found on intact unstimulated T cells in a monovalent form (one ligand-binding site per receptor) as well as in several distinct multivalent forms. This is in contrast to the TCR x CD3 stoichiometries determined by several biochemical means; however, these data can be explained by the effects of different detergents on the integrity of the receptor. Here, we discuss a model in which the multivalent receptors are important for the detection of low concentrations of ligand and therefore confer sensitivity, whereas the co-expressed monovalent TCR x CD3s allow a wide dynamic range.
Figures
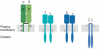




References
-
- Alarcón B, Gil D, Delgado P, Schamel WWA (2003) Initiation of TCR signaling: regulation within CD3 dimers. Immunol Rev 191: 38–46 - PubMed
-
- Anderson HA, Hiltbold EM, Roche PA (2000) Concentration of MHC class II molecules in lipid rafts facilitates antigen presentation. Nat Immunol 1: 156–162 - PubMed
-
- Bachmann MF, Speiser DE, Zakarian A, Ohashi PS (1998) Inhibition of TCR triggering by a spectrum of altered peptide ligands suggests the mechanism for TCR antagonism. Eur J Immunol 28: 3110–3119 - PubMed
-
- Blumberg RS, Alarcón B, Sancho J, McDermott FV, Lopez P, Breitmeyer J, Terhorst C (1990) Assembly and function of the T cell antigen receptor. Requirement of either the lysine or arginine residues in the transmembrane region of the α chain. J Biol Chem 265: 14036–14043 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

