Frontiers in pruritus research: scratching the brain for more effective itch therapy
- PMID: 16670758
- PMCID: PMC1451220
- DOI: 10.1172/JCI28553
Frontiers in pruritus research: scratching the brain for more effective itch therapy
Abstract
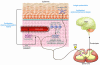
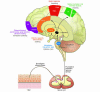
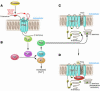
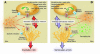
This Review highlights selected frontiers in pruritus research and focuses on recently attained insights into the neurophysiological, neuroimmunological, and neuroendocrine mechanisms underlying skin-derived itch (pruritogenic pruritus), which may affect future antipruritic strategies. Special attention is paid to newly identified itch-specific neuronal pathways in the spinothalamic tract that are distinct from pain pathways and to CNS regions that process peripheral pruritogenic stimuli. In addition, the relation between itch and pain is discussed, with emphasis on how the intimate contacts between these closely related yet distinct sensory phenomena may be exploited therapeutically. Furthermore, newly identified or unduly neglected intracutaneous itch mediators (e.g., endovanilloids, proteases, cannabinoids, opioids, neurotrophins, and cytokines) and relevant receptors (e.g., vanilloid receptor channels and proteinase-activated, cannabinoid, opioid, cytokine, and new histamine receptors) are discussed. In summarizing promising new avenues for managing itch more effectively, we advocate therapeutic approaches that strive for the combination of peripherally active antiinflammatory agents with drugs that counteract chronic central itch sensitization.
Figures

References
-
- Greaves M.W., Khalifa N. Itch: more than skin deep. Int. Arch. Allergy Immunol. 2004;135:166–172. - PubMed
-
- Yosipovitch G., Greaves M.W., Schmelz M. Itch. Lancet. 2003;361:690–694. - PubMed
-
- Biro T., et al. How best to fight that nasty itch - from new insights into the neuroimmunological, neuroendocrine, and neurophysiological bases of pruritus to novel therapeutic approaches. Exp. Dermatol. 2005;14:225–240. - PubMed
-
- Steinhoff M., et al. Neurophysiological, neuroimmunological and neuroendocrine basis of pruritus. J. Invest. Dermatol. 2006 In press. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

