Synthesis of ent-thallusin
- PMID: 16671797
- PMCID: PMC2518398
- DOI: 10.1021/ol0605777
Synthesis of ent-thallusin
Abstract
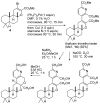
[reaction: see text] A three-step route from sclareol oxide (6) to bromo ester 4 in 53% overall yield was achieved using the efficient oxidation of an allylic bromide to an enal with bis(2,4,6-trimethylpyridine)silver(I) hexafluorophosphate in DMSO. Stille coupling of bromo ester 4 with stannylpyridine 5 gave the trimethyl ester of ent-thallusin in 54-92% yield by the stoichiometric conversion of 4 to a vinyl palladium intermediate prior to the addition of 5 to the reaction.
Figures
References
-
- Matsuo Y, Imagawa H, Nishizawa M, Shizuri Y. Science. 2005;307:1598. - PubMed
-
- Zimmermann N, Meggers E, Schultz PG. Bioorg. Chem. 2004;32:13–25. - PubMed
-
- Leite MAF, Sarragiotto MH, Imamura PM, Marsaioli AJ. J. Org. Chem. 1986;51:5409–5410.
-
- Barrero AF, Alvarez-Manzaneda EJ, Altarejos J, Salido S, Ramos JM. Tetrahedron. 1993;49:10405–10412.
-
- Aricu AN, Yu Andreeva, I., Vlad PF. Russ. Chem. Bull. 1996;45:2645–2648.
- Izvest. Akad. Nauk, Ser. Khim. 1996:2785–2789.
- Chem. Abstr. 1997;126:157649m.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous