Compensation by tumor suppressor genes during retinal development in mice and humans
- PMID: 16672052
- PMCID: PMC1481602
- DOI: 10.1186/1741-7007-4-14
Compensation by tumor suppressor genes during retinal development in mice and humans
Abstract
Background: The RB1 gene was the first tumor suppressor gene cloned from humans by studying genetic lesions in families with retinoblastoma. Children who inherit one defective copy of the RB1 gene have an increased susceptibility to retinoblastoma. Several years after the identification of the human RB1 gene, a targeted deletion of Rb was generated in mice. Mice with one defective copy of the Rb gene do not develop retinoblastoma. In this manuscript, we explore the different roles of the Rb family in human and mouse retinal development in order to better understand the species-specific difference in retinoblastoma susceptibility.
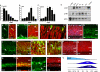
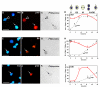
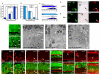
Results: We found that the Rb family of proteins (Rb, p107 and p130) are expressed in a dynamic manner during mouse retinal development. The primary Rb family member expressed in proliferating embryonic retinal progenitor cells in mice is p107, which is required for appropriate cell cycle exit during retinogenesis. The primary Rb family member expressed in proliferating postnatal retinal progenitor cells is Rb. p130 protein is expressed redundantly with Rb in postmitotic cells of the inner nuclear layer and the ganglion cell layer of the mouse retina. When Rb is inactivated in an acute or chronic manner during mouse retinal development, p107 is upregulated in a compensatory manner. Similarly, when p107 is inactivated in the mouse retina, Rb is upregulated. No changes in p130 expression were seen when p107, Rb or both were inactivated in the developing mouse retina. In the human retina, RB1 was the primary family member expressed throughout development. There was very little if any p107 expressed in the developing human retina. In contrast to the developing mouse retina, when RB1 was acutely inactivated in the developing human fetal retina, p107 was not upregulated in a compensatory manner.
Conclusion: We propose that intrinsic genetic compensation between Rb and p107 prevents retinoblastoma in Rb- or p107-deficient mice, but this compensation does not occur in humans. Together, these data suggest a model that explains why humans are susceptible to retinoblastoma following RB1 loss, but mice require both Rb and p107 gene inactivation.
Figures





Similar articles
-
Distinct patterns of expression of the RB gene family in mouse and human retina.Gene Expr Patterns. 2005 Jun;5(5):687-94. doi: 10.1016/j.modgep.2005.02.003. Epub 2005 Apr 9. Gene Expr Patterns. 2005. PMID: 15939381
-
Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice.Cell. 2007 Oct 19;131(2):378-90. doi: 10.1016/j.cell.2007.09.036. Cell. 2007. PMID: 17956737 Free PMC article.
-
Expression of the retinoblastoma family of tumor suppressors during murine embryonic orofacial development.Orthod Craniofac Res. 2003 Feb;6(1):32-47. doi: 10.1046/j.1439-0280.2003.2c035.x. Orthod Craniofac Res. 2003. PMID: 12627794
-
A new model of tumor susceptibility following tumor suppressor gene inactivation.Cell Cycle. 2008 Mar 15;7(6):735-40. doi: 10.4161/cc.7.6.5612. Epub 2008 Jan 11. Cell Cycle. 2008. PMID: 18239449 Review.
-
The RB protein family in retinal development and retinoblastoma: new insights from new mouse models.Dev Neurosci. 2004;26(5-6):417-34. doi: 10.1159/000082284. Dev Neurosci. 2004. PMID: 15855771 Review.
Cited by
-
Avoiding pitfalls of internal controls: validation of reference genes for analysis by qRT-PCR and Western blot throughout rat retinal development.PLoS One. 2012;7(8):e43028. doi: 10.1371/journal.pone.0043028. Epub 2012 Aug 20. PLoS One. 2012. PMID: 22916200 Free PMC article.
-
Osteosarcomagenesis: modeling cancer initiation in the mouse.Sarcoma. 2011;2011:694136. doi: 10.1155/2011/694136. Epub 2011 Feb 20. Sarcoma. 2011. PMID: 21403899 Free PMC article.
-
Rb substantially compensates for the double loss of p130 and p107 in adult but not embryonic neural stem cell lineages.Cell Death Dis. 2025 Jul 10;16(1):511. doi: 10.1038/s41419-025-07815-6. Cell Death Dis. 2025. PMID: 40640163 Free PMC article.
-
Histone Deacetylases in Retinoblastoma.Int J Mol Sci. 2024 Jun 24;25(13):6910. doi: 10.3390/ijms25136910. Int J Mol Sci. 2024. PMID: 39000021 Free PMC article. Review.
-
Childhood cancer and developmental biology a crucial partnership.Curr Top Dev Biol. 2011;94:1-13. doi: 10.1016/B978-0-12-380916-2.00001-2. Curr Top Dev Biol. 2011. PMID: 21295682 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

