Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias
- PMID: 16672364
- PMCID: PMC1472543
- DOI: 10.1073/pnas.0602133103
Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias
Abstract
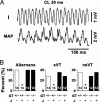
Catecholaminergic polymorphic ventricular tachycardia is a form of exercise-induced sudden cardiac death that has been linked to mutations in the cardiac Ca2+ release channel/ryanodine receptor (RyR2) located on the sarcoplasmic reticulum (SR). We have shown that catecholaminergic polymorphic ventricular tachycardia-linked RyR2 mutations significantly decrease the binding affinity for calstabin-2 (FKBP12.6), a subunit that stabilizes the closed state of the channel. We have proposed that RyR2-mediated diastolic SR Ca2+ leak triggers ventricular tachycardia (VT) and sudden cardiac death. In calstabin-2-deficient mice, we have now documented diastolic SR Ca2+ leak, monophasic action potential alternans, and bidirectional VT. Calstabin-deficient cardiomyocytes exhibited SR Ca2+ leak-induced aberrant transient inward currents in diastole consistent with delayed after-depolarizations. The 1,4-benzothiazepine JTV519, which increases the binding affinity of calstabin-2 for RyR2, inhibited the diastolic SR Ca2+ leak, monophasic action potential alternans and triggered arrhythmias. Our data suggest that calstabin-2 deficiency is as a critical mediator of triggers that initiate cardiac arrhythmias.
Conflict of interest statement
Conflict of interest statement: A.R.M. is on the scientific advisory board and owns shares in ARMGO Pharma, Inc., a start-up company that is developing JTV519 derivatives for clinical use in the treatment of heart failure and sudden cardiac death. S.E.L. and S.R. are consultants for ARMGO Pharma, Inc.
Figures





Similar articles
-
Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia.Circ Res. 2012 Aug 31;111(6):708-17. doi: 10.1161/CIRCRESAHA.112.273342. Epub 2012 Jul 24. Circ Res. 2012. PMID: 22828895 Free PMC article.
-
FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death.Cell. 2003 Jun 27;113(7):829-40. doi: 10.1016/s0092-8674(03)00434-3. Cell. 2003. PMID: 12837242
-
Defective domain-domain interactions within the ryanodine receptor as a critical cause of diastolic Ca2+ leak in failing hearts.Cardiovasc Res. 2009 Feb 15;81(3):536-45. doi: 10.1093/cvr/cvn303. Epub 2008 Nov 7. Cardiovasc Res. 2009. PMID: 18996969 Free PMC article.
-
Sarcoplasmic reticulum calcium leak and cardiac arrhythmias.Biochem Soc Trans. 2007 Nov;35(Pt 5):952-6. doi: 10.1042/BST0350952. Biochem Soc Trans. 2007. PMID: 17956253 Review.
-
Calstabin deficiency, ryanodine receptors, and sudden cardiac death.Biochem Biophys Res Commun. 2004 Oct 1;322(4):1267-79. doi: 10.1016/j.bbrc.2004.08.032. Biochem Biophys Res Commun. 2004. PMID: 15336974 Review.
Cited by
-
Targeted sarcoplasmic reticulum Ca2+ ATPase 2a gene delivery to restore electrical stability in the failing heart.Circulation. 2012 Oct 23;126(17):2095-104. doi: 10.1161/CIRCULATIONAHA.111.071480. Epub 2012 Sep 27. Circulation. 2012. PMID: 23019291 Free PMC article.
-
Susceptibility to Ventricular Arrhythmias Resulting from Mutations in FKBP1B, PXDNL, and SCN9A Evaluated in hiPSC Cardiomyocytes.Stem Cells Int. 2020 Sep 1;2020:8842398. doi: 10.1155/2020/8842398. eCollection 2020. Stem Cells Int. 2020. PMID: 32952569 Free PMC article.
-
Calcium cycling proteins and heart failure: mechanisms and therapeutics.J Clin Invest. 2013 Jan;123(1):46-52. doi: 10.1172/JCI62834. Epub 2013 Jan 2. J Clin Invest. 2013. PMID: 23281409 Free PMC article. Review.
-
Chain-reaction Ca(2+) signaling in the heart.J Clin Invest. 2007 Jul;117(7):1758-62. doi: 10.1172/JCI32496. J Clin Invest. 2007. PMID: 17607353 Free PMC article.
-
Cellular mechanisms of arrhythmogenic cardiac alternans.Prog Biophys Mol Biol. 2008 Jun-Jul;97(2-3):332-47. doi: 10.1016/j.pbiomolbio.2008.02.014. Epub 2008 Feb 15. Prog Biophys Mol Biol. 2008. PMID: 18395246 Free PMC article. Review.
References
-
- Lehnart S. E., Wehrens X. H., Laitinen P. J., Reiken S. R., Deng S. X., Cheng Z., Landry D. W., Kontula K., Swan H., Marks A. R. Circulation. 2004;109:3208–3214. - PubMed
-
- Laitinen P. J., Brown K. M., Piippo K., Swan H., Devaney J. M., Brahmbhatt B., Donarum E. A., Marino M., Tiso N., Viitasalo M., et al. Circulation. 2001;103:485–490. - PubMed
-
- Priori S. G., Napolitano C., Tiso N., Memmi M., Vignati G., Bloise R., Sorrentino V., Danieli G. A. Circulation. 2001;103:196–200. - PubMed
-
- De Rosa G., Delogu A. B., Piastra M., Chiaretti A., Bloise R., Priori S. G. Pediatr. Emerg. Care. 2004;20:175–177. - PubMed
-
- George C. H., Higgs G. V., Lai F. A. Circ. Res. 2003;93:531–540. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

