Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression
- PMID: 16672367
- PMCID: PMC1472541
- DOI: 10.1073/pnas.0511232103
Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression
Abstract
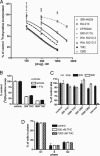
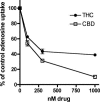
The plant-derived cannabinoids delta9-tetrahydrocannabinol (THC) and cannabidiol (CBD) both have immunosuppressive effects; although some effects of THC are mediated by the CB2 receptor, CB2 binds CBD weakly. In examining the effects of THC and CBD on microglial proliferation, we found that these compounds potently inhibit [3H]thymidine incorporation into a murine microglial cell line with no effect on cell cycle. Treatment with THC and CBD decreased [3H]thymidine uptake into microglia, with IC50 values that match inhibition of [3H]thymidine incorporation into DNA. CBD and, less potently, THC decreased uptake of [3H]adenosine to a similar extent as [3H]thymidine in both murine microglia and RAW264.7 macrophages. Binding studies confirm that CBD binds to the equilibrative nucleoside transporter 1 with a Ki < 250 nM. Because adenosine agonists have antiinflammatory effects, and because uptake of adenosine is a primary mechanism of terminating adenosine signaling, we tested the hypothesis that CBD is immunosuppressive because it enhances endogenous adenosine signaling. In vivo treatment with a low dose of CBD decreases TNFalpha production in lipopolysaccharide-treated mice; this effect is reversed with an A2A adenosine receptor antagonist and abolished in A2A receptor knockout mice. These studies demonstrate that CBD has the ability to enhance adenosine signaling through inhibition of uptake and provide a non-cannabinoid receptor mechanism by which CBD can decrease inflammation.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


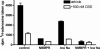


References
-
- Croxford J. L., Yamamura T. J. Neuroimmunol. 2005;166:3–18. - PubMed
-
- Wade D. T., Makela P., Robson P., House H., Bateman C. Mult. Scler. 2004;10:434–441. - PubMed
-
- Buckley N. E., McCoy K. L., Mezey E., Bonner T., Zimmer A., Felder C. C., Glass M. Eur. J. Pharmacol. 2000;396:141–149. - PubMed
-
- Mechoulam R., Parker L. A., Gallily R. J. Clin. Pharmacol. 2002;42:11S–19S. - PubMed
-
- Belgrave B. E., Bird K. D., Chesher G. B., Jackson D. M., Lubbe K. E., Starmer G. A., Teo R. K. Psychopharmacology (Berlin) 1979;64:243–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

