A unique fungal two-component system regulates stress responses, drug sensitivity, sexual development, and virulence of Cryptococcus neoformans
- PMID: 16672377
- PMCID: PMC1483045
- DOI: 10.1091/mbc.e06-02-0113
A unique fungal two-component system regulates stress responses, drug sensitivity, sexual development, and virulence of Cryptococcus neoformans
Abstract
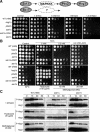
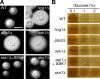
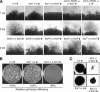
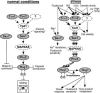
The stress-activated mitogen-activated protein kinase (MAPK) pathway is widely used by eukaryotic organisms as a central conduit via which cellular responses to the environment effect growth and differentiation. The basidiomycetous human fungal pathogen Cryptococcus neoformans uniquely uses the stress-activated Pbs2-Hog1 MAPK system to govern a plethora of cellular events, including stress responses, drug sensitivity, sexual reproduction, and virulence. Here, we characterized a fungal "two-component" system that controls these fundamental cellular functions via the Pbs2-Hog1 MAPK cascade. A typical response regulator, Ssk1, modulated all Hog1-dependent phenotypes by controlling Hog1 phosphorylation, indicating that Ssk1 is the major upstream signaling component of the Pbs2-Hog1 pathway. A second response regulator, Skn7, governs sensitivity to Na+ ions and the antifungal agent fludioxonil, negatively controls melanin production, and functions independently of Hog1 regulation. To control these response regulators, C. neoformans uses multiple sensor kinases, including two-component-like (Tco) 1 and Tco2. Tco1 and Tco2 play shared and distinct roles in stress responses and drug sensitivity through the Hog1 MAPK system. Furthermore, each sensor kinase mediates unique cellular functions for virulence and morphological differentiation. Our findings highlight unique adaptations of this global two-component MAPK signaling cascade in a ubiquitous human fungal pathogen.
Figures






References
-
- Aguilera J., Rodriguez-Vargas S., Prieto J. A. The HOG MAP kinase pathway is required for the induction of methylglyoxal-responsive genes and determines methylglyoxal resistance in Saccharomyces cerevisiae. Mol. Microbiol. 2005;56:228–239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

