Overflow metabolism in Escherichia coli during steady-state growth: transcriptional regulation and effect of the redox ratio
- PMID: 16672514
- PMCID: PMC1472329
- DOI: 10.1128/AEM.72.5.3653-3661.2006
Overflow metabolism in Escherichia coli during steady-state growth: transcriptional regulation and effect of the redox ratio
Abstract
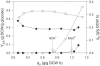
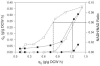
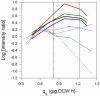
Overflow metabolism in the form of aerobic acetate excretion by Escherichia coli is an important physiological characteristic of this common industrial microorganism. Although acetate formation occurs under conditions of high glucose consumption, the genetic mechanisms that trigger this phenomenon are not clearly understood. We report on the role of the NADH/NAD ratio (redox ratio) in overflow metabolism. We modulated the redox ratio in E. coli through the expression of Streptococcus pneumoniae (water-forming) NADH oxidase. Using steady-state chemostat cultures, we demonstrated a strong correlation between acetate formation and this redox ratio. We furthermore completed genome-wide transcription analyses of a control E. coli strain and an E. coli strain overexpressing NADH oxidase. The transcription results showed that in the control strain, several genes involved in the tricarboxylic acid (TCA) cycle and respiration were repressed as the glucose consumption rate increased. Moreover, the relative repression of these genes was alleviated by expression of NADH oxidase and the resulting reduced redox ratio. Analysis of a promoter binding site upstream of the genes which correlated with redox ratio revealed a degenerate sequence with strong homology with the binding site for ArcA. Deletion of arcA resulted in acetate reduction and increased the biomass yield due to the increased capacities of the TCA cycle and respiration. Acetate formation was completely eliminated by reducing the redox ratio through expression of NADH oxidase in the arcA mutant, even at a very high glucose consumption rate. The results provide a basis for studying new regulatory mechanisms prevalent at reduced NADH/NAD ratios, as well as for designing more efficient bioprocesses.
Figures





References
-
- Andersen, K. B., and K. von Meyenburg. 1977. Charges of nicotinamide adenine nucleotides and adenylate energy charge as regulatory parameters of the metabolism in Escherichia coli. J. Biol. Chem. 252:4151-4156. - PubMed
-
- Auzat, I., S. Chapuy-Regaud, G. Le Bras, D. Dos Santos, A. D. Ogunniyi, I. Le Thomas, J. R. Garel, J. C. Paton, and M. C. Trombe. 1999. The NADH oxidase of Streptococcus pneumoniae: its involvement in competence and virulence. Mol. Microbiol. 34:1018-1028. - PubMed
-
- Bernofsky, C., and M. Swan. 1973. An improved cycling assay for nicotinamide adenine dinucleotide. Anal. Biochem. 53:452-458. - PubMed
-
- Berrios-Rivera, S. J., G. N. Bennett, and K. Y. San. 2002. Metabolic engineering of Escherichia coli: increase of NADH availability by overexpressing an NAD+-dependent formate dehydrogenase. Metabol. Eng. 4:217-229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

