Use of fluorescence resonance energy transfer for rapid detection of enteroviral infection in vivo
- PMID: 16672521
- PMCID: PMC1472337
- DOI: 10.1128/AEM.72.5.3710-3715.2006
Use of fluorescence resonance energy transfer for rapid detection of enteroviral infection in vivo
Abstract
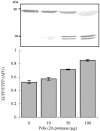
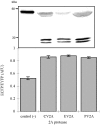
Enteroviruses can be easily transmitted through the fecal-oral route and cause a diverse array of clinical manifestations. Recent outbreaks associated with enteroviral contamination in aquatic environments have called for the development of a more efficient and accurate virus monitoring system. To develop a simple, rapid, and direct method for identifying enteroviral infections, we generated a fluorescent reporter system in which genetically engineered cells express a hybrid fluorescent indicator composed of a linker peptide, which is exclusively cleaved by the 2A protease (2A(pro)), flanked with a cyan fluorescent protein (CFP) and a yellow fluorescent protein undergoing fluorescence resonance energy transfer. The covalent linkage between two fluorophores is disrupted due to 2A(pro) activity upon viral infection, which results in an increase in CFP intensity. This allows the rapid (within 7.5 h) detection of very low numbers (10 PFU or fewer) of infectious enteroviruses.
Figures
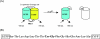


Similar articles
-
Real-time monitoring of human enterovirus (HEV)-infected cells and anti-HEV 3C protease potency by fluorescence resonance energy transfer.Antimicrob Agents Chemother. 2009 Feb;53(2):748-55. doi: 10.1128/AAC.00841-08. Epub 2008 Nov 17. Antimicrob Agents Chemother. 2009. PMID: 19015331 Free PMC article.
-
A fluorescence resonance energy transfer-based fluorometer assay for screening anti-coxsackievirus B3 compounds.J Virol Methods. 2011 Jan;171(1):176-82. doi: 10.1016/j.jviromet.2010.10.021. Epub 2010 Oct 26. J Virol Methods. 2011. PMID: 21029747
-
In vivo dynamics of enterovirus protease revealed by fluorescence resonance emission transfer (FRET) based on a novel FRET pair.Biochem Biophys Res Commun. 2007 Feb 23;353(4):939-45. doi: 10.1016/j.bbrc.2006.12.145. Epub 2006 Dec 27. Biochem Biophys Res Commun. 2007. PMID: 17207462
-
Enteroviral proteases: structure, host interactions and pathogenicity.Rev Med Virol. 2016 Jul;26(4):251-67. doi: 10.1002/rmv.1883. Epub 2016 May 4. Rev Med Virol. 2016. PMID: 27145174 Free PMC article. Review.
-
Drug discovery in enteroviral infections.Infect Disord Drug Targets. 2011 Jun;11(3):337-45. doi: 10.2174/187152611795768060. Infect Disord Drug Targets. 2011. PMID: 21488833 Review.
Cited by
-
Methods to detect infectious human enteric viruses in environmental water samples.Int J Hyg Environ Health. 2011 Nov;214(6):424-36. doi: 10.1016/j.ijheh.2011.07.014. Epub 2011 Sep 15. Int J Hyg Environ Health. 2011. PMID: 21920815 Free PMC article. Review.
-
Anti-enterovirus 71 effects of chrysin and its phosphate ester.PLoS One. 2014 Mar 5;9(3):e89668. doi: 10.1371/journal.pone.0089668. eCollection 2014. PLoS One. 2014. PMID: 24598537 Free PMC article.
-
A Cell-based Fluorescence Resonance Energy Transfer (FRET) Sensor Reveals Inter- and Intragenogroup Variations in Norovirus Protease Activity and Polyprotein Cleavage.J Biol Chem. 2015 Nov 13;290(46):27841-53. doi: 10.1074/jbc.M115.688234. Epub 2015 Sep 11. J Biol Chem. 2015. PMID: 26363064 Free PMC article.
-
Cell Cultures for Virology: Usability, Advantages, and Prospects.Int J Mol Sci. 2020 Oct 27;21(21):7978. doi: 10.3390/ijms21217978. Int J Mol Sci. 2020. PMID: 33121109 Free PMC article. Review.
-
Evolution of cyclic peptide protease inhibitors.Proc Natl Acad Sci U S A. 2011 Jul 5;108(27):11052-6. doi: 10.1073/pnas.1108045108. Epub 2011 Jun 20. Proc Natl Acad Sci U S A. 2011. PMID: 21690365 Free PMC article.
References
-
- Angres, B., and G. Green. April. 1999, posting date. Dual labeling using ECFP and EYFP in standard fluorescence microscopy. CLONTECHniques [Online.] http://www.clontech.com/clontech/archive/APR99UPD/dual.shtml.
-
- Banks, W., C. Klohe, and D. Battigelli. 2001. Occurrence and distribution of enteric viruses in shallow ground water and factors affecting well vulnerability to microbiological contamination in Worcester and Wicomico Counties, Maryland. U.S. Geological Survey water-resources investigations report 01-4147, p. 23. U.S. Geological Survey, Baltimore, Md.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

