A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels
- PMID: 16672657
- PMCID: PMC6674176
- DOI: 10.1523/JNEUROSCI.5080-05.2006
A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels
Abstract
Temperature transduction in mammals is possible because of the presence of a set of temperature-dependent transient receptor potential (TRP) channels in dorsal root ganglia neurons and skin cells. Six thermo-TRP channels, all characterized by their unusually high temperature sensitivity (Q10 > 10), have been cloned: TRPV1-4 are heat activated, whereas TRPM8 and TRPA1 are activated by cold. Because of the lack of structural information, the molecular basis for regulation by temperature remains unknown. In this study, we assessed the role of the C-terminal domain of thermo-TRPs and its involvement in thermal activation by using chimeras between the heat receptor TRPV1 and the cold receptor TRPM8, in which the entire C-terminal domain was switched. Here, we demonstrate that the C-terminal domain is modular and confers the channel phenotype regarding temperature sensitivity, channel gating kinetics, and PIP2 (phosphatidylinositol-4,5-bisphophate) modulation. Thus, thermo-TRP channels contain an interchangeable specific region, different from the voltage sensor, which allows them to sense temperature stimuli.
Figures
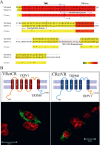
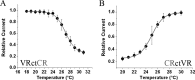


References
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824. - PubMed
-
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D (1999). A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398:436–441. - PubMed
-
- Choe S (2002). Potassium channel structures. Nat Rev Neurosci 3:115–121. - PubMed
-
- Chuang HH, Neuhausser WM, Julius D (2004). The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron 43:859–869. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
