Inhibition of feline (FIPV) and human (SARS) coronavirus by semisynthetic derivatives of glycopeptide antibiotics
- PMID: 16675038
- PMCID: PMC7114212
- DOI: 10.1016/j.antiviral.2006.03.005
Inhibition of feline (FIPV) and human (SARS) coronavirus by semisynthetic derivatives of glycopeptide antibiotics
Abstract
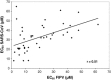
Various semisynthetic derivatives of glycopeptide antibiotics including vancomycin, eremomycin, teicoplanin, ristocetin A and DA-40926 have been evaluated for their inhibitory activity against feline infectious peritonitis virus (FIPV) and human (SARS-CoV, Frankfurt-1 strain) coronavirus in cell culture in comparison with their activity against human immunodeficiency virus (HIV). Several glycopeptide derivatives modified with hydrophobic substituents showed selective antiviral activity. For the most active compounds, the 50% effective concentrations (EC(50)) were in the lower micromolar range. In general, removal of the carbohydrate parts of the molecules did not affect the antiviral activity of the compounds. Some compounds showed inhibitory activity against both, whereas other compounds proved inhibitory to either, FIPV or SARS-CoV. There was no close correlation between the EC(50) values of the glycopeptide derivatives for FIPV or SARS-CoV.
Figures
References
-
- Balzarini J., Pannecouque C., De Clercq E., Pavlov A.Y., Printsevskaya S.S., Miroshnikova O.V., Reznikova M.I., Preobrazhenskaya M.N. Antiretroviral activity of semisynthetic derivatives of glycopeptide antibiotics. J. Med. Chem. 2003;46:2755–2764. - PubMed
-
- Barna C.J.C., Williams D.H., Williamson M.P. Structural features that affect the binding of teicoplanin, ristocetin A, and their derivatives to the bacterial cell-wall model N-acetyl-d-alanyl-d-alanine. J. Soc. Chem. Commun. 1985:254.
-
- Berdnikova T.F., Lomakina N.N., Olsufyeva E.N., Alexandrova L.G., Potapova N.P., Rozinov B.V., Malkova I.V., Orlova G.I. Structure and antimicrobial activity of products of partial degradation of antibiotic eremomycin. Antibiotics Chemother. (Russia) 1991;36:28–31. - PubMed
-
- Bognar R., Sztaricskai F., Munk M.E., Tamas J. Structure and stereochemistry of ristosamine. J. Org. Chem. 1974;39:2971–2974.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

