Post- and prejunctional consequences of ecto-ATPase inhibition: electrical and contractile studies in guinea-pig vas deferens
- PMID: 16675493
- PMCID: PMC1819469
- DOI: 10.1113/jphysiol.2006.109678
Post- and prejunctional consequences of ecto-ATPase inhibition: electrical and contractile studies in guinea-pig vas deferens
Abstract
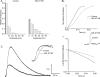
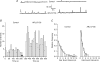
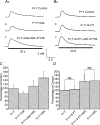
At sites of purinergic neurotransmission, synaptic ecto-ATPase is believed to limit the actions of ATP following its neural release. However, details of the modulation by this enzyme of the ATP-mediated conductance change and the possible mechanisms mediating this modulation remain unelucidated. We have addressed these issues by studying the effect of ARL 67156, a selective ecto-ATPase inhibitor, on ATP-mediated electrical and contractile activity in the sympathetically innervated guinea-pig vas deferens. ARL 67156 at 100 mum significantly potentiated the amplitude of spontaneous excitatory junction potentials (SEJPs) by 81.1% (P < 0.01) and prolonged their time courses (rise time by 49.7%, decay time constant by 38.2%; P < 0.01). Moreover, the frequency of occurrence of SEJPs was strikingly increased (from 0.28 +/- 0.13 to 0.90 +/- 0.26 Hz; P < 0.01), indicating an additional, primarily presynaptic, effect of ecto-ATPase inhibition. The frequency of occurrence of discrete events (DEs), which represent nerve stimulation-evoked quantal release of neurotransmitter, was also increased ( approximately 6-fold; P < 0.01), along with the appearance of DEs at previously 'silent' latencies. Purinergic contractions of the vas deferens were potentiated significantly (P < 0.01) by ARL 67156; these potentiated contractions were suppressed by the A1 agonist adenosine (P < 0.01) but left unaffected by the A1 antagonist 8-phenyltheophylline (8-PT). Our results indicate (i) that ecto-ATPase activity, in addition to modulating the ATP-mediated postjunctional conductance change, may regulate transmitter release prejunctionally under physiological conditions, and (ii) that the prejunctional regulation may be mediated primarily via presynaptic P2X, rather than A1, receptors.
Figures

Similar articles
-
The effect of P2 receptor antagonists and ATPase inhibition on sympathetic purinergic neurotransmission in the guinea-pig isolated vas deferens.Br J Pharmacol. 2000 Mar;129(6):1089-94. doi: 10.1038/sj.bjp.0703163. Br J Pharmacol. 2000. PMID: 10725256 Free PMC article.
-
Effects of cyclopiazonic acid on contractility and ecto-ATPase activity in guinea-pig urinary bladder and vas deferens.Br J Pharmacol. 1994 Nov;113(3):669-74. doi: 10.1111/j.1476-5381.1994.tb17044.x. Br J Pharmacol. 1994. PMID: 7858854 Free PMC article.
-
Enhancement of sympathetic purinergic neurotransmission in the guinea-pig isolated vas deferens by the novel ecto-ATPase inhibitor ARL 67156.Br J Pharmacol. 1996 Mar;117(5):867-72. doi: 10.1111/j.1476-5381.1996.tb15273.x. Br J Pharmacol. 1996. PMID: 8851503 Free PMC article.
-
ATP as a co-transmitter with noradrenaline in sympathetic nerves--function and fate.Ciba Found Symp. 1996;198:223-35; discussion 235-8. doi: 10.1002/9780470514900.ch13. Ciba Found Symp. 1996. PMID: 8879828 Review.
-
Modulation of synaptic activity in Purkinje neurons by ATP.Cerebellum. 2006;5(1):49-54. doi: 10.1080/14734220500497456. Cerebellum. 2006. PMID: 16527764 Review.
Cited by
-
Effects of carbenoxolone on syncytial electrical properties and junction potentials of guinea-pig vas deferens.Naunyn Schmiedebergs Arch Pharmacol. 2006 Dec;374(3):207-14. doi: 10.1007/s00210-006-0109-7. Epub 2006 Nov 9. Naunyn Schmiedebergs Arch Pharmacol. 2006. PMID: 17093918
-
Purinergic signalling in the reproductive system in health and disease.Purinergic Signal. 2014 Mar;10(1):157-87. doi: 10.1007/s11302-013-9399-7. Epub 2013 Nov 23. Purinergic Signal. 2014. PMID: 24271059 Free PMC article. Review.
-
Prejunctional and postjunctional actions of heptanol and 18 beta-glycyrretinic acid in the rodent vas deferens.Auton Neurosci. 2009 Jun 15;148(1-2):69-75. doi: 10.1016/j.autneu.2009.03.006. Epub 2009 Apr 16. Auton Neurosci. 2009. PMID: 19375392 Free PMC article.
-
Cellular function and molecular structure of ecto-nucleotidases.Purinergic Signal. 2012 Sep;8(3):437-502. doi: 10.1007/s11302-012-9309-4. Epub 2012 May 4. Purinergic Signal. 2012. PMID: 22555564 Free PMC article. Review.
References
-
- Bennett MR, Gibson WG. On the contribution of quantal secretion from close-contact and loose-contact varicosities to the synaptic potentials in the vas deferens. Phil Trans Roy Soc Lond B Biol Sci. 1995;347:187–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

