Structure of the multidrug transporter EmrD from Escherichia coli
- PMID: 16675700
- PMCID: PMC3152482
- DOI: 10.1126/science.1125629
Structure of the multidrug transporter EmrD from Escherichia coli
Erratum in
- Science. 2007 Sep 21;317(5845):1682
Abstract
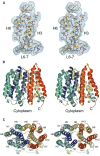
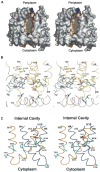
EmrD is a multidrug transporter from the Major Facilitator Superfamily that expels amphipathic compounds across the inner membrane of Escherichia coli. Here, we report the x-ray structure of EmrD determined to a resolution of 3.5 angstroms. The structure reveals an interior that is composed mostly of hydrophobic residues, which is consistent with its role transporting amphipathic molecules. Two long loops extend into the inner leaflet side of the cell membrane. This region can serve to recognize and bind substrate directly from the lipid bilayer. We propose that multisubstrate specificity, binding, and transport are facilitated by these loop regions and the internal cavity.
Figures

References
-
- Naroditskaya V, Schlosser MJ, Fang NY, Lewis K. Biochemical and Biophysical Research Communications. 1993;196:803. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

