Haemophilus influenzae forms biofilms on airway epithelia: implications in cystic fibrosis
- PMID: 16675778
- PMCID: PMC2662906
- DOI: 10.1164/rccm.200509-1459OC
Haemophilus influenzae forms biofilms on airway epithelia: implications in cystic fibrosis
Abstract
Rationale: Nontypeable Haemophilus influenzae (NTHi) commonly infects patients with cystic fibrosis (CF), especially early in childhood. Bacteria biofilms are increasingly recognized as contributing to bacterial persistence and disease pathogenesis in CF.
Objectives: This study investigated ability of NTHi to form biofilms and its impact on airway epithelia using in vivo and in vitro analyses.
Methods: We evaluated bronchoalveolar lavage fluid from young patients with CF for evidence of NTHi biofilms. To further investigate the pathogenesis of NTHi in respiratory infections, we developed a novel in vitro coculture model of NTHi biofilm formation on polarized human airway epithelial cells grown at the air-liquid interface.
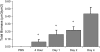
Measurements and main results: In bronchoalveolar lavage fluid samples from young, asymptomatic patients with CF, we found morphologic evidence suggestive of NTHi biofilm formation. In addition, 10 clinical NTHi isolates from patients with CF formed biofilms on plastic surfaces. NTHi formed biofilms on the apical surface of cultured airway epithelia. These biofilms exhibited decreased susceptibility to antibiotics and were adherent to epithelial surfaces. Airway epithelial cells remained viable throughout 4 d of coculture, and responded to NTHi with nuclear factor-kappaB signaling, and increased chemokine and cytokine secretion.
Conclusions: NTHi formed adherent biofilms on the apical surface airway epithelia with decreased susceptibility to antibiotics, and respiratory cells exhibited inflammatory and host defense responses-evidence of a dynamic host-pathogen interaction. The data presented here have implications both for understanding early CF lung disease pathogenesis and for the treatment of early, asymptomatic colonization of patients with CF with H. influenzae.
Figures





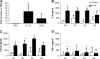
References
-
- Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 2000;407:762–764. - PubMed
-
- Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol 2003;57:677–701. - PubMed
-
- Jesaitis AJ, Franklin MJ, Berglund D, Sasaki M, Lord CI, Bleazard JB, Duffy JE, Beyenal H, Lewandowski Z. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J Immunol 2003;171:4329–4339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

