Apoptosis regulation in tetraploid cancer cells
- PMID: 16675948
- PMCID: PMC1478174
- DOI: 10.1038/sj.emboj.7601127
Apoptosis regulation in tetraploid cancer cells
Abstract
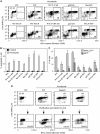
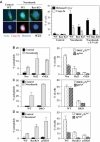
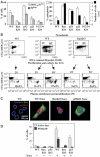
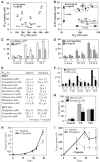
Tetraploidy can result in cancer-associated aneuploidy. As shown here, freshly generated tetraploid cells arising due to mitotic slippage or failed cytokinesis are prone to undergo Bax-dependent mitochondrial membrane permeabilization and subsequent apoptosis. Knockout of Bax or overexpression of Bcl-2 facilitated the survival of tetraploid cells at least as efficiently as the p53 or p21 knockout. When tetraploid cells were derived from diploid p53 and Bax-proficient precursors, such cells exhibited an enhanced transcription of p53 target genes. Tetraploid cells exhibited an enhanced rate of spontaneous apoptosis that could be suppressed by inhibition of p53 or by knockdown of proapoptotic p53 target genes such as BBC3/Puma, GADD45A and ferredoxin reductase. Unexpectedly, tetraploid cells were more resistant to DNA damaging agents (cisplatin, oxaliplatin and camptothecin) than their diploid counterparts, and this difference disappeared upon inhibition of p53 or knockdown of p53-inducible ribonucleotide reductase. Tetraploid cells were also more resistant against UVC and gamma-irradiation. These data indicate the existence of p53-dependent alterations in apoptosis regulation in tetraploid cells.
Figures


References
-
- Adams JM (2003) Ways of dying: multiple pathways to apoptosis. Genes Dev 17: 2481–2495 - PubMed
-
- Barrett MT, Pritchard D, Palanca-Wessels C, Anderson J, Reid BJ, Rabinovitch PS (2003) Molecular phenotype of spontaneously arising 4N (G2-tetraploid) intermediates of neoplastic progression in Barrett's esophagus. Cancer Res 63: 4211–4217 - PubMed
-
- Bourdon JC, Deguin-Chambon V, Lelong JC, Dessen P, May P, Debuire B, May E (1997) Further characterisation of the p53 responsive element—identification of new candidate genes for trans-activation by p53. Oncogene 14: 85–94 - PubMed
-
- Castedo M, Ferri K, Roumier T, Metivier D, Zamzami N, Kroemer G (2002a) Quantitation of mitochondrial alterations associated with apoptosis. J Immunol Methods 265: 39–47 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

